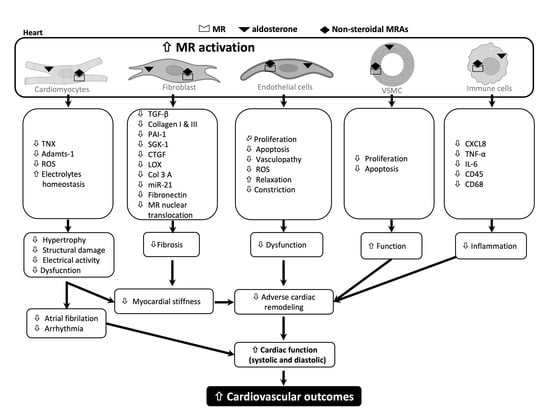
Potential Impact of Non-Steroidal Mineralocorticoid Receptor Antagonists in Cardiovascular Disease
Abstract
:1. Introduction
2. Chemical Properties of Steroidal and Non-Steroidal MRAs
3. Pharmacological Effects of Steroidal and Non-Steroidal MRAs in CVD: Evidence from Pre-Clinical Studies
3.1. Blood Pressure
| Salt-Induced Hypertensive Animal Models | |||||||||||
| MRAs | Animal Model | Drug Dosage | Blood Pressure | Cardiac Remodeling | Cardiac Function | Vascular Changes | Molecular Mechanism | Ref. | |||
| Eplerenone | DSS rats; 8% NaCl diet for 12 weeks | 10, 30, 100 mg/kg/day 7 weeks | ⇩ SBP (8.5% for 100 mg/kg/day) | ⇩ LVW/BW (32%, 39% and 44%) * ⇩ LVEDD (18%, 19%, 21%) * ⇧ LVES | ⇧ % FS (20%, 22%, 25%) * ⇩ HR (13%, 9.6%, 13.2%) * | ⇩ Wall:lumen ratio (38%, 46%, 43%) * | Oxidative stress | Fibrosis | Other | [26] | |
| ⇩ NADPH oxidase activity (26%, 31%, 37% *) ⇩ p22phox ⇩ p47phox ⇩ gp91phox | ⇩ Perivascular fibrosis (31%, 40%, 54% *) ⇧ SERCA2 ⇩ TGF-β1 ⇩ Collagen-I | ⇧ P-eNOS ⇧ P-Akt ⇧ Nitrite production | |||||||||
| DSS rats; 8% NaCl diet for 7 weeks | 10, 30, 100 mg/kg/day 7 weeks | ⇩ SBP (9% for 100 mg/kg/day) | ⇩ LVW/BW (10%, 10%, 13%) * ⇨ BNP (8% ↓ for 100 mg/kg) | - | - | Other pathologies | [19] | ||||
| ⇩ Urinary protein ⇩ Glomerulosclerosis (100 mg/kg) ⇨Tubular injury | |||||||||||
| Spironolactone | DSS rats 8% NaCl diet for 7 weeks | 10, 30, 100 mg/kg/day; 7 weeks | ⇩ SBP (10% for 100 mg/kg/day) | ⇩ LVW/BW (9%, and 12% for 30 and 100 mg/kg/day) ⇨ BNP (19% ↓ for 100 mg/kg) | - | - | Other pathologies | [19] | |||
| ⇨ Urinary protein excretion ⇩ Glomerulosclerosis (100 mg/kg) | |||||||||||
| Esaxerenone | DSS rats 8% NaCl diet for 7 weeks | 0.25, 0.5, 1 and 2 mg/kg; 7 weeks | ⇩ SBP (6%, 12%, 18% and 27%) * | ⇩ LVW/BW (13%, 19%, 26% for 0.5, 1 and 2 mg/kg/day, respectively) ⇩ BNP (38% and 43% ↓ for 1 and 2 mg/kg, respectively) | - | - | - | [19] | |||
| DSS rats 8% NaCl diet for 10 weeks | 1 mg/kg/day (0.001% esaxerenone w/w); 4 weeks | ⇩ SBP (9%) | ⇩ HW/BW (8%) ⇩ LVW/BW (8%) ⇩ NPPA ⇩ NPPB ⇩ MYH7 ⇩ LVIDs (13%) ⇩ ESV (33%) | ⇧ EF (16%) ⇧ FS (25%) ⇧ SV (19%) ⇧ CO (26%) ⇨ HR | - | Oxidative stress | Inflammation | Fibrosis | [27] | ||
| ⇩ 4-HNE ⇩ gp47phox ⇩ p22phox ⇩ MDA | ⇩ CXCL8 ⇩ TNF-α ⇩ IL-6 | ⇩ TGF-β ⇩ Collagen I and III ⇩ PAI-1 ⇩ SGK-1 | |||||||||
| Finerenone | SD rats, UNX, DOCA/salt | 0.1, 1, and 10 mg/kg/day; 10 weeks | ⇩ SBP (10 mg/kg) | ⇩ HW/BW (1 and 10 mg/kg) ⇩ pro-BNP (1 and 10 mg/kg) ⇩ Structural heart injury (10 mg/kg) | ⇩ Focal vasculopathy (10 mg/kg) | - - ⇩ Fibrosis | [21] | ||||
| Myocardial/Vascular Injury Model | |||||||||||
| MRAs | Model | Drug Dosage | Blood Pressure | Cardiac Remodeling | Cardiac Function | Vascular Changes | Molecular Mechanism | Ref. | |||
| Systolic | Diastolic | Oxidative Stress | Inflammation | Fibrosis | |||||||
| Eplerenone | Wistar rats, coronary artery ligation (MI) | 100 mg/kg/day | - | ⇨ HW/BW ⇨ pro-BNP | ⇨ dP/dtmax ⇨ dP/dtmin ⇨ HR | ⇨ LVEDP ⇨ Tau | - | - | - | ⇨ Osteopontin (mRNA) | [21] |
| Finerenone | 0.1, 0.3, and 1 mg/kg/day, 8 weeks | - | ⇨ HW/BW ⇩ pro-BNP | ⇧ dP/dtmax ⇩ dP/dtmin (1 mg/kg) ⇨ HR | ⇘ LVEDP ⇘ Tau (for 1 mg/kg) | - | - | - | ⇩ Osteopontin (mRNA) | ||
| Eplerenone | C57BL/6, transverse aortic constriction (TAC) | 200 mg/kg/day, 4 weeks | - | ⇨ LVM ⇨ IVS ⇨ LVPW ⇘ BNP ⇨ Tnnt2 | ⇨ EF ⇨ HR | - | - | - | - | ⇨ Fibrosis | [31] |
| Finerenone | 10 mg/kg/day, 4 weeks | - | ⇩ LVM ⇩ IVS ⇩ LVPW ⇩ BNP ⇩ Tnnt2 (mRNA) | ⇨ EF ⇩ HR | - | - | - | - | ⇨ Fibrosis | ||
| Finerenone | C57BL/6, artery injury model | 1 and 10 mg/kg/day, 10 days | - | - | - | - | ⇧ Re-endothelialization ⇩ Ki-67 ⇩ α-SMA ⇩ Neointimal lesion formation | - | ⇩ CD45 | - | [32] |
| Eplerenone | 129/Sv mice, isoproterenol 25 mg/kg for 4 days | 200 mg/kg/day | ⇩ SBP (12.5%) | ⇩ HW/TL ⇩ LVM/TL ⇩ GLS | ⇘ EF ⇨ ESV | ⇨ EDV ⇩ LVAWd ⇩ LVPWd | - | ⇩ NADPH oxidase 1 | ⇨ CD 68 | ⇘ TNX ⇩ TGF-β ⇩ Col1a1 ⇘ Gal3 | [33] |
| Finerenone | 10 mg/kg/day | ⇘ SBP (6.7%) | ⇩ HW/TL ⇩ LVM/TL ⇩ GLS | ⇘ EF ⇩ ESV | ⇨ EDV ⇩ LVAWd ⇩ LVPWd | - | ⇩NADPH oxidase 1 | ⇩ CD 68 | ⇩ TNX ⇩ TGF-β ⇩ Col1a1 ⇩ Gal3 | ||
| Transgenic Hypertensive Model | |||||||||||
| MRAs | Model | Drug Dosage | Blood Pressure | Cardiac Remodeling | Cardiac Function | Vascular Changes | Molecular Mechanism | Ref. | |||
| Finerenone | Renin-transgenic (mRen2)27 rats, L-NAME | 1 and 3 mg/kg; 7 weeks | ⇩ SBP | ⇨ Pro-BNP | - | ⇘ Vasculopathy ⇘ Vascular fibrosis | ⇘ Fibrosis | [28] | |||
| RacET transgenic mice | 100 ppm with chow; 5 months | - | ⇨ HW ⇨ LVW ⇩ LAW ⇩ LA/LV | Systolic | Diastolic | - | Oxidative stress | Fibrosis | Other | [34] | |
| ⇨ FS ⇗ EF ⇨ LVDs ⇘ LVESV ⇘ SV | ⇨ LVDs ⇘ LVEDV ⇨ E/e′ ratio | ⇩ NADPH oxidase activity | ⇩ TGF-β ⇩ CTGF ⇩ LOX ⇩ Osteopontin ⇩ COL3A1 | ⇩ MR nuclear translocation | |||||||
| MWF rats | 10 mg/kg/day; 4 weeks | ⇩ SBP | - | - | - | ⇧ Ach-induced relaxation ⇩ NA-induced constriction ⇩ AngII-induced constriction | ⇧ p47phox ⇧ Mn-SOD ⇧ Cu/Zn-SOD ⇧ P-eNOS | - | ⇩ UAE | [29] | |
| Metabolic Syndrome Model | |||||||||||
| MRAs | Model | Drug Dosage | Blood Pressure | Cardiac Remodeling | Cardiac Function | Vascular Changes | Molecular mechanism | Ref. | |||
| Systolic | Diastolic | Oxidative Stress | Inflammation | Fibrosis | |||||||
| Spironolactone | C57BL6J (female), Western diet with high fat | 1 mg/kg/day; 7 days | ⇨ SBP ⇨ DBP ⇨ MAP | ⇩ LV weight ⇧ sarcomere lengths | ⇩ LVDs ⇨ LVESP ⇨ LVESPVR | ⇧ E′/A′ ⇨ E/e′ ⇩ IVRT ⇩ Diastolic relaxation time | - | ⇩ ROS | ⇩ M1 MΦ ⇧ M2 MΦ | ⇨ Interstitial fibrosis | [35] |
| Finerenone | Zucker fatty fa/fa rats | 2 mg/kg; 7 days | ⇨ SBP | ⇨ LVW ⇧ LV tissue perfusion | ⇩ LVDs ⇨ LVESP ⇨ LVESPVR ⇧ FS | ⇨ LVDd ⇘ LVEDP ⇩ LVEDPVR ⇩ Tau | ⇨ TPR | Oxidative stress | Fibrosis | Other | [36] |
| ⇩ LV ROS ⇩ Plasma nitrite | ⇨ Collagen | ⇘ Proteinuria | |||||||||
| 2 mg/kg; 90 days | ⇨ SBP | ⇩ LVW ⇧LV tissue perfusion | ⇩ LVDs ⇨ LVESP ⇨ LVESPVR ⇧ FS | ⇩ LVDd ⇩ LVEDP ⇩ LVEDPVR ⇘ Tau | ⇨ TPR | - | ⇩ Collagen | ⇩ Proteinuria | |||
| Finerenone | OVX (at 4 month) | 1 mg/kg/day (from 7 months); 1 month | ⇩ SBP (13%) ⇨ DBP | ⇨ HW/TL ⇨ LVW/TL ⇨ MCSA ⇨ Capillary density | ⇨ LVESP ⇨ LVESPVR ⇨ dP/dtmin ⇨ FS ⇨ CO | ⇩ LVEDP ⇩ LVEDPVR ⇨ Tau | ⇧ Vascular relaxation | Oxidative stress | Fibrosis | Inflammation | [30] |
| ⇨ LV ROS ⇨ IFM ROS ⇨ SSM ROS | ⇨ Col-I ⇨ Col-III | PECAM-1+ vessels | |||||||||
| Nephrectomy Model | |||||||||||
| MRAs | Model | Drug Dosage | Blood Pressure | Cardiac Remodeling | Cardiac Function | Vascular Changes | Molecular Mechanism | Ref. | |||
| Systolic | Diastolic | ||||||||||
| Finerenone | UNX+ DOCA/salt | 0.1, 1 and 10 mg/kg/day; 10 weeks | ⇩ SBP (10 mg/kg) | ⇩ HW/BW ⇩ pro-BNP (1 and 10 mg) ⇩ Structural heart injury (10 mg/kg) | - | - | ⇩ Focal vasculopathy (10 mg/kg) | ⇩ Fibrosis | [21] | ||
| Finerenone | Subtotal Nx; B6D2 male | 2.5 mg/kg/d with chow; 6 weeks | ⇨ SBP ⇩ DBP | ⇩ HW/TL ⇨ Cardiomyocytes cross-sectional area ⇘ nppb (BNP) | ⇗ HR ⇗ EF ⇧ FS ⇨ SV ⇨ CO | ⇧ E/A ratio ⇨ dP/dtmax ⇨ dP/dtmin ⇨ Tau | - | ⇘ Interstitial fibrosis ⇩ α-SMA ⇧ NOV | [37] | ||
3.2. Myocardial Structural Remodeling
3.3. Myocardial Function
3.4. Vascular Remodeling and Function
4. Molecular Mechanisms Underlying the Beneficial Effects of Non-Steroidal MRAs on CVD: Evidence from Pre-Clinical Studies
4.1. Oxidative Stress
4.2. Inflammation
4.3. Interstitial Fibrosis
4.4. Vascular Injury
5. Pharmacological Effects of Non-Steroidal MRAs on Cardiovascular Outcomes: Evidence from Clinical Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Slivnick, J.; Lampert, B.C. Hypertension and Heart Failure. Heart Fail. Clin. 2019, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Hajouli, S.; Ludhwani, D. Heart Failure And Ejection Fraction; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553115/ (accessed on 20 December 2022).
- Chaudhry, S.I.; McAvay, G.; Chen, S.; Whitson, H.; Newman, A.B.; Krumholz, H.M.; Gill, T.M. Risk Factors for Hospitalization Among Older Persons Newly Diagnosed with Heart Failure: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2013, 61, 635. [Google Scholar] [CrossRef] [Green Version]
- Bauersachs, J.; Opez-Andrés, N.L.; Opez Andrés, N.L.; Translational, C.; Navarrabiomed, R. Mineralocorticoid receptor in cardiovascular diseases—Clinical trials and mechanistic insights. Br. J. Pharmacol. 2021, 179, 3119–3134. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Funder, J.W. The pathophysiology of aldosterone in the cardiovascular system. Ann. N. Y. Acad. Sci. 2002, 970, 89–100. [Google Scholar] [CrossRef]
- Gekle, M.; Grossmann, C. Actions of aldosterone in the cardiovascular system: The good, the bad, and the ugly? Pflugers Arch. 2009, 458, 231–246. [Google Scholar] [CrossRef]
- Ertram, B.; Itt, P.; Aiez, F.; Annad, Z.; Emme, I.J.R.; Obert, R.; Ody, C.; Lain, A.; Astaigne, C.; Lfonso, A.; et al. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Edelmann, F.; Wachter, R.; Schmidt, A.G.; Kraigher-Krainer, E.; Colantonio, C.; Kamke, W.; Duvinage, A.; Stahrenberg, R.; Durstewitz, K.; Löffler, M.; et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA 2013, 309, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Savill, P. Spironolactone in heart failure with preserved ejection fraction. Practitioner 2014, 258, 10. [Google Scholar]
- Barrera-Chimal, J.; Kolkhof, P.; Lima-Posada, I.; Joachim, A.; Rossignol, P.; Jaisser, F. Differentiation between emerging non-steroidal and established steroidal mineralocorticoid receptor antagonists: Head-to-head comparisons of pharmacological and clinical characteristics. Expert Opin. Investig. Drugs 2021, 30, 1141–1157. [Google Scholar] [CrossRef]
- Young, M.J.; Kanki, M.; Karthigan, N.; Konstandopoulos, P. The Role of the Mineralocorticoid Receptor and Mineralocorticoid Receptor–Directed Therapies in Heart Failure. Endocrinology 2021, 162, bqab105. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Jaisser, F.; Kim, S.Y.; Filippatos, G.; Nowack, C.; Pitt, B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: Comparison at bench and bedside. Handb. Exp. Pharmacol. 2017, 243, 271–305. [Google Scholar]
- Garthwaite, S.M.; McMahon, E.G. The evolution of aldosterone antagonists. Mol. Cell. Endocrinol. 2004, 217, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bădilă, E. The expanding class of mineralocorticoid receptor modulators: New ligands for kidney, cardiac, vascular, systemic and behavioral selective actions. Acta Endocrinol. 2020, 16, 487. [Google Scholar]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Homma, T.; Morikawa, Y.; Ubukata, N.; Tsuruoka, H.; Aoki, K.; Ishikawa, H.; Mizuno, M.; Sada, T. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur. J. Pharmacol. 2015, 761, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Tsuruoka, H.; Homma, T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur. J. Pharmacol. 2015, 769, 266–273. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Ghanim, H.; Arora, P. Improving the residual risk of renal and cardiovascular outcomes in diabetic kidney disease: A review of pathophysiology, mechanisms, and evidence from recent trials. Diabetes Obes. Metab. 2022, 24, 365–376. [Google Scholar] [CrossRef]
- Kolkhof, P.; Delbeck, M.; Kretschmer, A.; Steinke, W.; Hartmann, E.; Bärfacker, L.; Eitner, F.; Albrecht-Küpper, B.; Schäfer, S. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J. Cardiovasc. Pharmacol. 2014, 64, 69–78. [Google Scholar] [CrossRef]
- Yamada, M.; Takei, M.; Suzuki, E.; Takakusa, H.; Kotsuma, M.; Washio, T.; Murayama, N.; Inoue, S.-I.; Izumi, T. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica 2017, 47, 1090–1103. [Google Scholar] [CrossRef]
- Gerisch, M.; Heinig, R.; Engelen, A.; Lang, D.; Kolkhof, P.; Radtke, M.; Platzek, J.; Lovis, K.; Rohde, G.; Schwarz, T. Biotransformation of Finerenone, a Novel Nonsteroidal Mineralocorticoid Receptor Antagonist, in Dogs, Rats, and Humans, In Vivo and In Vitro. Drug Metab. Dispos. 2018, 46, 1546–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M.; Ubukata, O.; Homma, T.; Asoh, Y.; Honzumi, M.; Hayashi, N.; Saito, K.; Tsuruoka, H.; Aoki, K.; Hanzawa, H. Crystal structure of the mineralocorticoid receptor ligand-binding domain in complex with a potent and selective nonsteroidal blocker, esaxerenone (CS-3150). FEBS Lett. 2020, 594, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Yoshida, K.; Nakano, S.; Ohno, T.; Honda, T.; Tsubokou, Y.; Matsuoka, H. Cardioprotective mechanisms of eplerenone on cardiac performance and remodeling in failing rat hearts. Hypertension 2006, 47, 671–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Sawano, T.; Sen, A.; Hossain, A.; Jahan, N.; Kobara, H.; Masaki, T.; Kosaka, S.; Kitada, K.; Nakano, D.; et al. Cardioprotective effects of a nonsteroidal mineralocorticoid receptor blocker, esaxerenone, in dahl salt-sensitive hypertensive rats. Int. J. Mol. Sci. 2021, 22, 2069. [Google Scholar] [CrossRef]
- Kolkhof, P.; Hartmann, E.; Freyberger, A.; Pavkovic, M.; Mathar, I.; Sandner, P.; Droebner, K.; Joseph, A.; Hüser, J.; Eitner, F. Effects of Finerenone Combined with Empagliflozin in a Model of Hypertension-Induced End-Organ Damage. Transl. Res. Res. Artic. Am. J. Nephrol. 2021, 52, 642–652. [Google Scholar] [CrossRef]
- González-Blázquez, R.; Somoza, B.; Gil-Ortega, M.; Ramos, M.M.; Ramiro-Cortijo, D.; Vega-Martín, E.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Kreutz, R.; et al. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front. Pharmacol. 2018, 9, 1131. [Google Scholar] [CrossRef] [Green Version]
- Pieronne-Deperrois, M.; Guéret, A.; Djerada, Z.; Crochemore, C.; Harouki, N.; Henry, J.P.; Dumesnil, A.; Larchevêque, M.; do Rego, J.C.; do Rego, J.L.; et al. Mineralocorticoid receptor blockade with finerenone improves heart function and exercise capacity in ovariectomized mice. ESC Heart Fail. 2021, 8, 1933–1943. [Google Scholar] [CrossRef]
- Grune, J.; Benz, V.; Brix, S.; Salatzki, J.; Blumrich, A.; Höft, B.; Klopfleisch, R.; Foryst-Ludwig, A.; Kolkhof, P.; Kintscher, U. Steroidal and Nonsteroidal Mineralocorticoid Receptor Antagonists Cause Differential Cardiac Gene Expression in Pressure Overload-induced Cardiac Hypertrophy. J. Cardiovasc. Pharmacol. 2016, 67, 402–411. [Google Scholar] [CrossRef]
- Lavall, D.; Jacobs, N.; Mahfoud, F.; Kolkhof, P.; Böhm, M.; Laufs, U. The non-steroidal mineralocorticoid receptor antagonist finerenone prevents cardiac fibrotic remodeling. Biochem. Pharmacol. 2019, 168, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Fliegner, D.; Schubert, C.; Penkalla, A.; Witt, H.; Kararigas, G.; Dworatzek, E.; Staub, E.; Martus, P.; Noppinger, P.R.; Kintscher, U.; et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1597–R1606. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Beyhoff, N.; Smeir, E.; Chudek, R.; Blumrich, A.; Ban, Z.; Brix, S.; Betz, I.R.; Schupp, M.; Foryst-Ludwig, A.; et al. Selective Mineralocorticoid Receptor Cofactor Modulation as Molecular Basis for Finerenone’s Antifibrotic Activity. Hypertension 2018, 71, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Hay, I.; Zhang, G.; Maurer, M.; Wang, J.; Burkhoff, D. Development of heart failure in chronic hypertensive Dahl rats: Focus on heart failure with preserved ejection fraction. Hypertension 2006, 47, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Lachaux, M.; Barrera-Chimal, J.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Dumesnil, A.; Wecker, D.; Richard, V.; Kolkhof, P.; Jaisser, F.; et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes. Obes. Metab. 2018, 20, 2399–2407. [Google Scholar] [CrossRef]
- Bonnard, B.; Pieronne-Deperrois, M.; Djerada, Z.; Elmoghrabi, S.; Kolkhof, P.; Ouvrard-Pascaud, A.; Mulder, P.; Jaisser, F.; Messaoudi, S. Mineralocorticoid receptor antagonism improves diastolic dysfunction in chronic kidney disease in mice. J. Mol. Cell. Cardiol. 2018, 121, 124–133. [Google Scholar] [CrossRef]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.R.; Zornoff, L.A.M. Cardiac Remodeling: Concepts, Clinical Impact, PathophysiologicalMechanisms and Pharmacologic Treatment. Arq. Bras. Cardiol. 2016, 106, 62. [Google Scholar] [CrossRef]
- Díez, J.; González, A.; López, B.; Querejeta, R. Mechanisms of disease: Pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 209–216. [Google Scholar] [CrossRef]
- Lother, A.; Fürst, D.; Bergemann, S.; Gilsbach, R.; Grahammer, F.; Huber, T.B.; Hilgendorf, I.; Bode, C.; Moser, M.; Hein, L. Deoxycorticosterone Acetate/Salt-Induced Cardiac But Not Renal Injury Is Mediated By Endothelial Mineralocorticoid Receptors Independently From Blood Pressure. Hypertension 2016, 67, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Dutzmann, J.; Musmann, R.J.; Haertlé, M.; Daniel, J.M.; Sonnenschein, K.; Schäfer, A.; Kolkhof, P.; Bauersachs, J.; Sedding, D.G. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS ONE 2017, 12, e0184888. [Google Scholar] [CrossRef] [Green Version]
- Queisser, N.; Schupp, N. Aldosterone, oxidative stress, and NF-κB activation in hypertension-related cardiovascular and renal diseases. Free Radic. Biol. Med. 2012, 53, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Murase, T.; Takatsu, M.; Matsuura, N.; Nagasawa, K.; Hattori, T.; Watanabe, S.; Murohara, T.; Nagata, K. Roles of oxidative stress and the mineralocorticoid receptor in cardiac pathology in a rat model of metabolic syndrome. Nagoya J. Med. Sci. 2015, 77, 275. [Google Scholar] [PubMed]
- Briones, A.M.; Touyz, R.M. Aldosterone/MR Signaling, Oxidative Stress, and Vascular Dysfunction. In Aldosterone-Mineralocorticoid Receptor-Cell Biology to Translational Medicine; IntechOpen Limited: London, UK, 2019; ISBN 978-1-83962-199-4. [Google Scholar] [CrossRef]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: Role of small GTPase Rac1. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhang, Y.Y.; Frieler, R.A.; Zheng, X.J.; Zhang, W.C.; Sun, X.N.; Yang, Q.Z.; Ma, S.M.; Huang, B.; Berger, S.; et al. Myeloid Mineralocorticoid Receptor Deficiency Inhibits Aortic Constriction-Induced Cardiac Hypertrophy in Mice. PLoS ONE 2014, 9, e110950. [Google Scholar] [CrossRef] [PubMed]
- Usher, M.G.; Duan, S.Z.; Ivaschenko, C.Y.; Frieler, R.A.; Berger, S.; Schütz, G.; Lumeng, C.N.; Mortensen, R.M. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J. Clin. Investig. 2010, 120, 3350–3364. [Google Scholar] [CrossRef] [Green Version]
- Rickard, A.J.; Morgan, J.; Bienvenu, L.A.; Fletcher, E.K.; Cranston, G.A.; Shen, J.Z.; Reichelt, M.E.; Delbridge, L.M.; Young, M.J. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension 2012, 60, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Bene, N.C.; Alcaide, P.; Wortis, H.H.; Jaffe, I.Z. Mineralocorticoid Receptors in Immune Cells; Emerging Role in Cardiovascular Disease. Steroids 2014, 91, 38. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Chimal, J.; Estrela, G.R.; Lechner, S.M.; Giraud, S.; El Moghrabi, S.; Kaaki, S.; Kolkhof, P.; Hauet, T.; Jaisser, F. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018, 93, 1344–1355. [Google Scholar] [CrossRef]
- Rafatian, N.; Westcott, K.V.; White, R.A.; Leenen, F.H.H. Cardiac macrophages and apoptosis after myocardial infarction: Effects of central MR blockade. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R879–R887. [Google Scholar] [CrossRef] [Green Version]
- Amador, C.A.; Barrientos, V.; Peña, J.; Herrada, A.A.; González, M.; Valdés, S.; Carrasco, L.; Alzamora, R.; Figueroa, F.; Kalergis, A.M.; et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 2014, 63, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Luettges, K.; Bode, M.; Thiele, A.; Ritter, D.; Klopfleisch, R.; Kappert, K.; Foryst-Ludwig, A.; Kolkhof, P.; Wenzel, U.; Kintscher, U. Finerenone reduces renal RORgt gd T-Cells and protects against cardiorenal damage. Am. J. Nephrol. 2022, 53, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Le Billan, F.; Perrot, J.; Carceller, E.; Travers, S.; Viengchareun, S.; Kolkhof, P.; Lombès, M.; Fagart, J. Antagonistic effects of finerenone and spironolactone on the aldosterone-regulated transcriptome of human kidney cells. FASEB J. 2021, 35, e21314. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.; Morgan, J.; Funder, J.W.; Fuller, P.J.; Young, M.J. Mediators of mineralocorticoid receptor-induced profibrotic inflammatory responses in the heart. Clin. Sci. 2009, 116, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Tesch, G.H.; Young, M.J. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front. Pharmacol. 2017, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Gao, L.; Niu, Y.; Zhou, Y.; Lin, H.; Jiang, J.; Kong, X.; Liu, X.; Li, L. Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biol. Pharm. Bull. 2015, 38, 1591–1598. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Biwer, L.A.; Moss, M.E.; Man, J.J.; Aronovitz, M.J.; Martin, G.L.; Carrillo-Salinas, F.J.; Salvador, A.M.; Alcaide, P.; Jaffe, I.Z. Mineralocorticoid Receptor in Smooth Muscle Contributes to Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2021, 14, 217–231. [Google Scholar] [CrossRef]
- McGraw, A.P.; Bagley, J.; Chen, W.S.; Galayda, C.; Nickerson, H.; Armani, A.; Caprio, M.; Carmeliet, P.; Jaffe, I.Z. Aldosterone Increases Early Atherosclerosis and Promotes Plaque Inflammation Through a Placental Growth Factor-Dependent Mechanism. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2013, 2, e000018. [Google Scholar] [CrossRef] [Green Version]
- Scicchitano, P.; Milo, M.; Mallamaci, R.; De Palo, M.; Caldarola, P.; Massari, F.; Gabrielli, D.; Colivicchi, F.; Ciccone, M.M. Inclisiran in lipid management: A Literature overview and future perspectives. Biomed. Pharmacother. 2021, 143, 112227. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Vega-Martín, E.; Martín-Ramos, M.; González-Blázquez, R.; Pulido-Olmo, H.; Ruiz-Hurtado, G.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Somoza, B.; et al. Finerenone Reduces Intrinsic Arterial Stiffness in Munich Wistar Frömter Rats, a Genetic Model of Chronic Kidney Disease. Am. J. Nephrol. 2020, 51, 294–303. [Google Scholar] [CrossRef]
- Iwahana, T.; Saito, Y.; Okada, S.; Kato, H.; Ono, R.; Kobayashi, Y. Safety and efficacy of esaxerenone in Japanese hypertensive patients with heart failure with reduced ejection fraction: A retrospective study. PLoS ONE 2021, 16, e0259485. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Williams, B. Nocturnal Hypertension and Heart Failure: Mechanisms, Evidence, and New Treatments. Hypertension 2021, 78, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Hoshide, S.; Kanegae, H.; Kario, K. Cardiovascular Event Risks Associated with Masked Nocturnal Hypertension Defined by Home Blood Pressure Monitoring in the J-HOP Nocturnal Blood Pressure Study. Hypertension 2020, 76, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yamakawa, S. Effect of esaxerenone on nocturnal blood pressure and natriuretic peptide in different dipping phenotypes. Hypertens. Res. 2022, 45, 97–105. [Google Scholar] [CrossRef]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Böhm, M.; Gheorghiade, M.; Køber, L.; Krum, H.; Maggioni, A.P.; Ponikowski, P.; Voors, A.A.; Zannad, F.; et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 2016, 37, 2105–2114. [Google Scholar] [CrossRef] [Green Version]
- Sato, N.; Ajioka, M.; Yamada, T.; Kato, M.; Myoishi, M.; Yamada, T.; Kim, S.Y.; Nowack, C.; Kolkhof, P.; Shiga, T. A Randomized Controlled Study of Finerenone vs. Eplerenone in Japanese Patients With Worsening Chronic Heart Failure and Diabetes and/or Chronic Kidney Disease. Circ. J. 2016, 80, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Alonso, A.; Lopez, F.L.; Matsushita, K.; Loehr, L.R.; Agarwal, S.K.; Chen, L.Y.; Soliman, E.Z.; Astor, B.C.; Coresh, J. Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011, 123, 2946–2953. [Google Scholar] [CrossRef] [Green Version]
- Rolfes, C.D.; Howard, S.A.; Goff, R.P.; Iaizzo, P.A. Cardiac remodeling as a consequence of atrial fibrillation: An anatomical study of perfusion-fixed human heart specimens. J. Geriatr. Cardiol. 2011, 8, 141. [Google Scholar] [PubMed] [Green Version]
- Filippatos, G.; Bakris, G.L.; Pitt, B.; Agarwal, R.; Rossing, P.; Ruilope, L.M.; Butler, J.; Lam, C.S.P.; Kolkhof, P.; Roberts, L.; et al. Finerenone Reduces New-Onset Atrial Fibrillation in Patients With Chronic Kidney Disease and Type 2 Diabetes. J. Am. Coll. Cardiol. 2021, 78, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Shikata, K.; Nangaku, M.; Okuda, Y.; Sawanobori, T. Efficacy and Safety of Esaxerenone (CS-3150) for the Treatment of Type 2 Diabetes with Microalbuminuria: A Randomized, Double-Blind, Placebo-Controlled, Phase II Trial. Clin. J. Am. Soc. Nephrol. 2019, 14, 1161–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostick, B.; Habibi, J.; DeMarco, V.G.; Jia, G.; Domeier, T.L.; Lambert, M.D.; Aroor, A.R.; Nistala, R.; Bender, S.B.; Garro, M.; et al. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1126–H1135. [Google Scholar] [CrossRef]
| In Vitro Model | ||||
|---|---|---|---|---|
| MRAs | Cells/Model | Drug Dosage | Molecular Mechanism | Ref. |
| Eplerenone | H9C2/MR+ cardiomyocytes, aldosterone 1, 10, 100 nmol/L | 0.05, 0.5, 5, 50 µM | ⇩ TNX ⇩ Adamts-1 | [33] |
| Finerenone | 0.05, 0.5, 5 µM | ⇩ TNX ⇩ Adamts-1 | ||
| Finerenone | Neonatal cardiac fibroblast; aldosterone 10 nM, 24 h/Ang II 1 µM, 3 h | 500 nm for 25 h | ⇩ CTGF ⇩ TGF-β ⇩ LOX ⇩ miR-21 ⇩ Fibronectin ⇩ MR nuclear translocation | [34] |
| Finerenone | Human coronary artery smooth muscle cells, aldosterone 10, 20, 50 nM, 24 h | 1 and 10 nM | ⇩ Proliferation ⇘ Apoptosis | [32] |
| Human umbilical vein endothelial cells, aldosterone 10, 20, 50 nM, 24 h | ⇩ Proliferation ⇩Apoptosis | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.; Jahan, N.; Rahman, M.T.; Nishiyama, A. Potential Impact of Non-Steroidal Mineralocorticoid Receptor Antagonists in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1922. https://doi.org/10.3390/ijms24031922
Rahman A, Jahan N, Rahman MT, Nishiyama A. Potential Impact of Non-Steroidal Mineralocorticoid Receptor Antagonists in Cardiovascular Disease. International Journal of Molecular Sciences. 2023; 24(3):1922. https://doi.org/10.3390/ijms24031922
Chicago/Turabian StyleRahman, Asadur, Nourin Jahan, Md Tanvir Rahman, and Akira Nishiyama. 2023. "Potential Impact of Non-Steroidal Mineralocorticoid Receptor Antagonists in Cardiovascular Disease" International Journal of Molecular Sciences 24, no. 3: 1922. https://doi.org/10.3390/ijms24031922
APA StyleRahman, A., Jahan, N., Rahman, M. T., & Nishiyama, A. (2023). Potential Impact of Non-Steroidal Mineralocorticoid Receptor Antagonists in Cardiovascular Disease. International Journal of Molecular Sciences, 24(3), 1922. https://doi.org/10.3390/ijms24031922