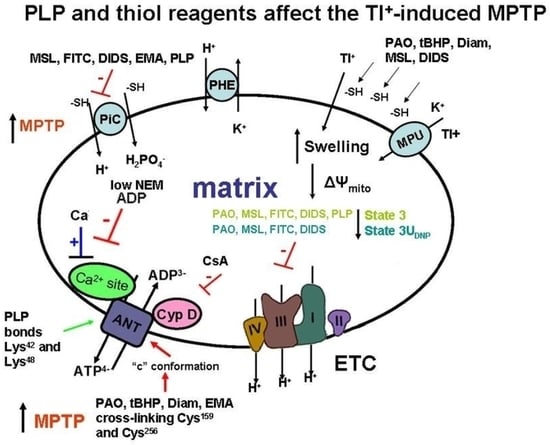
A Comparative Study on the Effects of the Lysine Reagent Pyridoxal 5-Phosphate and Some Thiol Reagents in Opening the Tl+-Induced Mitochondrial Permeability Transition Pore
Abstract
:1. Introduction
2. Results
2.1. Effects of PLP and Thiol Reagents on the Tl+–Induced Swelling of Succinate-Energized Rat Liver Mitochondria
2.2. Effects of Tl+ and Thiol-Modifying Agents on Respiration and ΔΨmito of Succinate-Energized Rat Liver Mitochondria
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Mitochondrial Isolation
4.4. Swelling of Mitochondria
4.5. Oxygen Consumption Assay
4.6. Mitochondrial Membrane Potential
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3UDNP state | 2,4-Dinitrophenol uncoupled state |
| ΔΨmito | Inner mitochondrial membrane potential |
| ANT | Adenine nucleotide translocase |
| CAT | Carboxyatractyloside |
| CsA | Cyclosporine A |
| CyP-D | Cyclophilin D |
| Diam | Diamide |
| DIDS | 4,4′-Diisothiocyanostilbene-2,2′-disulfonate |
| DNP | 2,4-Dinitrophenol |
| EGTA | Ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid |
| EMA | Eosin-5-maleimide |
| FITC | Fluorescein isothiocyanate |
| IMM | Inner mitochondrial membrane |
| MPTP | Mitochondrial permeability transition pore |
| MSL | Mersalyl |
| NEM | n-Ethylmaleimide |
| PAO | Phenylarsine oxide |
| PiC | Mitochondrial phosphate carrier |
| Pi | Inorganic phosphate |
| PLP | Pyridoxal 5-phosphate |
| RCR | Respiratory control ratio |
| RLM | Reactive oxygen species |
| tBHP | Tert-butyl hydroperoxide |
References
- Bernardi, P.; Carraro, M.; Lippe, G. The mitochondrial permeability transition: Recent progress and open questions. FEBS Lett. 2021, 289, 7051–7074. [Google Scholar] [CrossRef]
- Brustovetsky, N. The role of adenine nucleotide translocase in the mitochondrial permeability transition. Cells 2020, 9, 2686. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The c ring of the F1Fo ATP synthase forms the mitochondrial permeability transition pore: A critical appraisal. Front. Oncol. 2014, 4, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotkov, S.M.; Saris, N.E. Influence of Tl+ on mitochondrial permeability transition pore in Ca2+-loaded rat liver mitochondria. J. Bioenerg. Biomembr. 2011, 43, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, S.M. Mitochondria as a Key Intracellular Target of Thallium Toxicity; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2022; pp. 1–264. [Google Scholar]
- Korotkov, S.M.; Konovalova, S.A.; Brailovskaya, I.V.; Saris, N.E. To involvement the conformation of the adenine nucleotide translocase in opening the Tl+-induced permeability transition pore in Ca2+-loaded rat liver mitochondria. Toxicol. Vitr. 2016, 32, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Korotkov, S.M.; Konovalova, S.A.; Nesterov, V.P.; Brailovskaya, I.V. Mersalyl prevents the Tl+-induced permeability transition pore opening in the inner membrane of Ca2+-loaded rat liver mitochondria. Biochem. Biophys. Res. Commun. 2018, 495, 1716–1721. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Konovalova, S.A.; Brailovskaya, I.V. Diamide accelerates opening of the Tl+-induced permeability transition pore in Ca2+-loaded rat liver mitochondria. Biochim. Biophys. Res. Commun. 2015, 468, 360–364. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Novozhilov, A.V. The joint influence of Tl+ and thiol-modifying agents on rat liver mitochondrial parameters in vitro. Int. J. Mol. Sci. 2022, 23, 8964. [Google Scholar] [CrossRef]
- McStay, G.P.; Clarke, S.J.; Halestrap, A.P. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J. 2002, 367, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majima, E.; Koike, H.; Hong, Y.M.; Shinohara, Y.; Terada, H. Characterization of cysteine residues of mitochondrial ADP/ATP carrier with the SH-reagents eosin 5-maleimide and N-ethylmaleimide. J. Biol. Chem. 1993, 268, 22181–22187. [Google Scholar] [CrossRef]
- Halestrap, A.P. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010, 38, 841–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petronilli, V.; Costantini, P.; Scorrano, L.; Colonna, R.; Passamonti, S.; Bernardi, P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J. Biol. Chem. 1994, 269, 16638–16642. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Brenner, C. The adenine nucleotide translocase: A central part of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem. 2003, 10, 1507–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nantes, I.L.; Rodrigues, T.; Caires, A.C.; Cunha, R.L.; Pessoto, F.S.; Yokomizo, C.H.; Araujo-Chaves, J.C.; Faria, P.A.; Santana, D.P.; dos Santos, C.G. Specific effects of reactive thiol drugs on mitochondrial bioenergetics. J. Bioenerg. Biomembr. 2011, 43, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Majima, E.; Shinohara, Y.; Yamaguchi, N.; Hong, Y.M.; Terada, H. Importance of loops of mitochondrial ADP/ATP carrier for its transport activity deduced from reactivities of its cysteine residues with the sulfhydryl reagent eosin-5-maleimide. Biochemistry 1994, 33, 9530–9536. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, A.; Petronilli, V.; Bernardi, P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry 1993, 32, 4461–4465. [Google Scholar] [CrossRef]
- Eriksson, O.; Fontaine, E.; Bernardi, P. Chemical modification of arginines by 2,3-butanedione and phenylglyoxal causes closure of the mitochondrial permeability transition pore. J. Biol. Chem. 1998, 273, 12669–12674. [Google Scholar] [CrossRef] [Green Version]
- Antoniel, M.; Giorgio, V.; Fogolari, F.; Glick, G.D.; Bernardi, P.; Lippe, G. The oligomycin-sensitivity conferring protein of mitochondrial ATP synthase: Emerging new roles in mitochondrial pathophysiology. Int. J. Mol. Sci. 2014, 15, 7513–7536. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, O.; Fontaine, E.; Petronilli, V.; Bernardi, P. Inhibition of the mitochondrial cyclosporin A-sensitive permeability transition pore by the arginine reagent phenylglyoxal. FEBS Lett. 1997, 409, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, J.W. Intracellular trafficking of the pyridoxal cofactor. Implications for health and metabolic disease. Arch. Biochem. Biophys. 2015, 592, 20–26. [Google Scholar] [CrossRef]
- Tragni, V.; Primiano, G.; Tummolo, A.; Beltrame, L.C.; La Piana, G.; Sgobba, M.N.; Cavalluzzi, M.M.; Paterno, G.; Gorgoglione, R.; Volpicella, M.; et al. Personalized medicine in mitochondrial health and disease: Molecular basis of therapeutic approaches based on nutritional supplements and their analogs. Molecules 2022, 27, 3494. [Google Scholar] [CrossRef] [PubMed]
- Means, G.E.; Feeney, R.E. Affinity labeling of pancreatic ribonuclease. J. Biol. Chem. 1971, 246, 5532–5533. [Google Scholar] [CrossRef] [PubMed]
- Bogner, W.; Aquila, H.; Klingenberg, M. The transmembrane arrangement of the ADP/ATP carrier as elucidated by the lysine reagent pyridoxal 5-phosphate. Eur. J. Biochem. 1986, 161, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.S.; Ma, J.; Hart, G.W. Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc. Natl. Acad. Sci. USA 2015, 112, 6050–6055. [Google Scholar] [CrossRef] [Green Version]
- Kunk, C.; Kruger, J.; Mendoza, G.; Markitan, J.; Bias, T.; Mann, A.; Nath, A.; Geldenhuys, W.J.; Menze, M.A.; Konkle, M.E. MitoNEET’s reactivity of Lys55 toward pyridoxal phosphate demonstrates its activity as a transaminase enzyme. ACS Chem. Biol. 2022, 17, 2716–2722. [Google Scholar] [CrossRef]
- Stipani, I.; Mangiullo, G.; Stipani, V.; Daddabbo, L.; Natuzzi, D.; Palmieri, F. Inhibition of the reconstituted mitochondrial oxoglutarate carrier by arginine-specific reagents. Arch. Biochem. Biophys. 1996, 331, 48–54. [Google Scholar] [CrossRef]
- Gremse, D.A.; Dean, B.; Kaplan, R.S. Effect of pyridoxal 5′-phosphate on the function of the purified mitochondrial tricarboxylate transport protein. Arch. Biochem. Biophys. 1995, 316, 215–219. [Google Scholar] [CrossRef]
- Tahiliani, A.G.; Keene, T.; Kaplan, R.S. Characterization of the inhibitor sensitivity of the coenzyme A transport system in isolated rat heart mitochondria. J. Bioenerg. Biomembr. 1992, 24, 635–640. [Google Scholar] [CrossRef]
- Majima, E.; Ishida, M.; Miki, S.; Shinohara, Y.; Terada, H. Specific labeling of the bovine heart mitochondrial phosphate carrier with fluorescein 5-isothiocyanate: Roles of Lys185 and putative adenine nucleotide recognition site in phosphate transport. J. Biol. Chem. 2001, 276, 9792–9799. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, L.; Lasorsa, F.M.; De Palma, A.; Palmieri, F.; Runswick, M.J.; JWalker, E. Identification of the yeast ACR1 gene product as a succinate-fumarate transporter essential for growth on ethanol or acetate. FEBS Lett. 1997, 417, 114–118. [Google Scholar] [CrossRef]
- Palmieri, L.; Vozza, A.; Agrimi, G.; De Marco, V.; Runswick, M.J.; Palmieri, F.; Walker, J.E. Identification of the yeast mitochondrial transporter for oxaloacetate and sulfate. J. Biol. Chem. 1999, 274, 22184–22190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, L.; Agrimi, G.; Runswick, M.J.; Fearnley, I.M.; Palmieri, F.; Walker, J.E. Identification in Saccharomyces cerevisiae of two isoforms of a novel mitochondrial transporter for 2-oxoadipate and 2-oxoglutarate. J. Biol. Chem. 2001, 276, 1916–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiermonte, G.; Palmieri, L.; Dolce, V.; Lasorsa, F.M.; Palmieri, F.; Runswick, M.J.; Walker, J.E. The sequence, bacterial expression, and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and Caenorhabditis elegans. J. Biol. Chem. 1998, 273, 24754–24759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Aguilar, M.; Baines, C.P. Physiological and pathological roles of mitochondrial SLC25 carriers. Biochem. J. 2013, 45, 371–386. [Google Scholar] [CrossRef] [Green Version]
- Scalera, V.; Giangregorio, N.; De Leonardis, S.; Console, L.; Carulli, E.S.; Tonazzi, A. Characterization of a novel mitochondrial ascorbate transporter from rat liver and potato mitochondria. Front. Mol. Biosci. 2018, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Natuzzi, D.; Daddabbo, L.; Stipani, V.; Cappello, A.R.; Miniero, D.V.; Capobianco, L.; Stipani, I. Inactivation of the reconstituted oxoglutarate carrier from bovine heart mitochondria by pyridoxal 5′-phosphate. J. Bioenerg. Biomembr. 1999, 31, 535–541. [Google Scholar] [CrossRef]
- Indiveri, C.; Abruzzo, G.; Stipani, I.; Palmieri, F. Identification and purification of the reconstitutively active glutamine carrier from rat kidney mitochondria. Biochem. J. 1998, 333, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Stappen, R.; Dierks, T.; Bröer, A.; Krämer, R. Probing the active site of the reconstituted aspartate/glutamate carrier from mitochondria. Structure/function relationship involving one lysine and two cysteine residues. Eur. J. Biochem. 1992, 210, 269–277. [Google Scholar] [CrossRef]
- Dierks, T.; Krämer, R. Asymmetric orientation of the reconstituted aspartate/glutamate carrier from mitochondria. Biochim. Biophys. Acta 1988, 937, 112–126. [Google Scholar] [CrossRef]
- Stappen, R.; Krämer, R. Functional properties of the reconstituted phosphate carrier from bovine heart mitochondria: Evidence for asymmetric orientation and characterization of three different transport modes. Biochim. Biophys. Acta 1993, 1149, 40–48. [Google Scholar] [CrossRef]
- Stepp, L.R.; Reed, L.J. Active-site modification of mammalian pyruvate dehydrogenase by pyridoxal 5′-phosphate. Biochemistry 1985, 24, 7187–7191. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hatefi, Y. Mitochondrial nicotinamide nucleotide transhydrogenase: Inhibition by ethoxyformic anhydride, dansyl chloride, and pyridoxal phosphate. Arch. Biochem. Biophys. 1985, 243, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.J.; Mo, T.; Sawyers, D.L.; Harrison, J.H. Biphasic inactivation of procine heart mitochondrial malate dehydrogenase by pyridoxal 5′-phosphate. J. Biol. Chem. 1975, 250, 710–715. [Google Scholar] [CrossRef]
- Chen, S.S.; Engel, P.C. Reversible modification of pig heart mitochondrial malate dehydrogenase by pyridoxal 5′-phosphate. Biochem. J. 1975, 151, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Le-Quoc, K.; Le-Quoc, D. Involvement of the ADP/ATP carrier in calcium-induced perturbations of the mitochondrial inner membrane permeability: Importance of the orientation of the nucleotide binding site. Arch. Biochem. Biophys. 1988, 265, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Le-Quoc, D.; Le-Quoc, K. Relationships between the NAD(P) redox state, fatty acid oxidation, and inner membrane permeability in rat liver mitochondria. Arch. Biochem. Biophys. 1989, 273, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Lui, A.; Lumeng, L.; Li, T.K. Transport of pyridoxine and pyridoxal 5′-phosphate in isolated rat liver mitochondria. J. Biol. Chem. 1982, 257, 14903–14906. [Google Scholar] [CrossRef]
- Whittaker, M.M.; Penmatsa, A.; Whittaker, J.W. The Mtm1p carrier and pyridoxal 5′-phosphate cofactor trafficking in yeast mitochondria. Arch. Biochem. Biophys. 2015, 568, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Korotkov, S.M. Effects of Tl+ on the inner membrane thiol groups, respiration, and swelling in succinate-energized rat liver mitochondria were modified by thiol reagents. Biometals 2021, 34, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Takano, T.; Ohkuma, S. Similarity of lysosomal H+-ATPase to mitochondrial F0F1-ATPase in sensitivity to anions and drugs as revealed by solubilization and reconstitution. Biochim. Biophys. Acta 1986, 854, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Tagaya, M.; Futai, M. Modification of gastric (H+ + K+)-ATPase with pyridoxal 5′-phosphate. J. Biol. Chem. 1988, 263, 3652–3656. [Google Scholar] [CrossRef] [PubMed]
- Komatsu-Takaki, M. Effects of energization and substrates on the reactivities of lysine residues of the chloroplast ATP synthase beta subunit. Eur. J. Biochem. 1995, 228, 265–270. [Google Scholar] [CrossRef]
- Tzeng, C.M.; Hsu, L.H.; Pan, R.L. Inhibition of tonoplast ATPase from etiolated mung bean seedlings by fluorescein 5′-isothiocyanate. Biochem. J. 1992, 285, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Bogner, W.; Aquila, H. ADP/ATP carrier: Analysis of transmembrane folding using pyridoxal phosphate. Methods Enzymol. 1986, 125, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Houstĕk, J.; Pedersen, P.L. Adenine nucleotide and phosphate transport systems of mitochondria. Relative location of sulfhydryl groups based on the use of the novel fluorescent probe eosin-5-maleimide. J. Biol. Chem. 1985, 260, 6288–6295. [Google Scholar] [CrossRef] [PubMed]
- Waldmeier, P.C.; Feldtrauer, J.J.; Qian, T.; Lemasters, J.J. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporine derivative NIM811. Mol. Pharmacol. 2002, 62, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buelna-Chontal, M.; Pavón, N.; Correa, F.; Hernández-Esquivel, L.; Chávez, E. Titration of lysine residues on adenine nucleotide translocase by fluorescamine induces permeability transition. Cell Biol. Int. 2014, 38, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.; Ferguson, S.J. Identification of an essential beta chain lysine residue from bovine heart mitochondrial ATPase specifically modified with nitrobenzofurazan. FEBS Lett. 1985, 179, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Cunningham, D.; Cross, R.L.; Senior, A.E. Pyridoxal 5′-diphospho-5′-adenosine binds at a single site on isolated alpha-subunit from Escherichia coli F1-ATPase and specifically reacts with lysine 201. J. Biol. Chem. 1988, 263, 5640–5645. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotkov, S.M.; Novozhilov, A.V. A Comparative Study on the Effects of the Lysine Reagent Pyridoxal 5-Phosphate and Some Thiol Reagents in Opening the Tl+-Induced Mitochondrial Permeability Transition Pore. Int. J. Mol. Sci. 2023, 24, 2460. https://doi.org/10.3390/ijms24032460
Korotkov SM, Novozhilov AV. A Comparative Study on the Effects of the Lysine Reagent Pyridoxal 5-Phosphate and Some Thiol Reagents in Opening the Tl+-Induced Mitochondrial Permeability Transition Pore. International Journal of Molecular Sciences. 2023; 24(3):2460. https://doi.org/10.3390/ijms24032460
Chicago/Turabian StyleKorotkov, Sergey M., and Artemy V. Novozhilov. 2023. "A Comparative Study on the Effects of the Lysine Reagent Pyridoxal 5-Phosphate and Some Thiol Reagents in Opening the Tl+-Induced Mitochondrial Permeability Transition Pore" International Journal of Molecular Sciences 24, no. 3: 2460. https://doi.org/10.3390/ijms24032460
APA StyleKorotkov, S. M., & Novozhilov, A. V. (2023). A Comparative Study on the Effects of the Lysine Reagent Pyridoxal 5-Phosphate and Some Thiol Reagents in Opening the Tl+-Induced Mitochondrial Permeability Transition Pore. International Journal of Molecular Sciences, 24(3), 2460. https://doi.org/10.3390/ijms24032460