CB2 Receptor as Emerging Anti-Inflammatory Target in Duchenne Muscular Dystrophy
Abstract
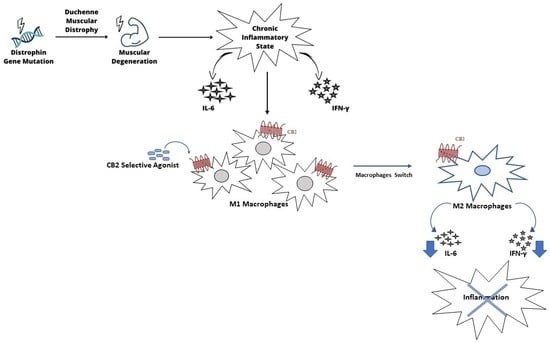
:1. Introduction
2. Results
2.1. Evaluation of Inflammatory State: Pro- and Anti-Inflammatory Cytokines Release and Iron Internalization
2.2. Macrophage Phenotype Characterization
2.3. CB2 Receptor Expression Level in DMD-Associated Macrophages
2.4. Effect of CB2 Receptor Stimulation on Inflammatory State
2.5. Effect of CB2 Receptor Stimulation on Macrophage Polarization
3. Discussion
4. Materials and Methods
4.1. Source of Macrophages
4.2. Macrophages Primary Cultures
4.3. Drugs and Treatments
4.4. Protein Isolation and Western Blotting
4.5. ELISA
4.6. Iron Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dowling, J.J.; Weihl, C.C.; Spencer, M.J. Molecular and cellular basis of genetically inherited skeletal muscle disorders. Nat. Rev. Mol. Cell Biol. 2021, 22, 713–732. [Google Scholar] [CrossRef]
- Tidball, J.G.; Welc, S.S.; Wehling-Henricks, M. Immunobiology of Inherited Muscular Dystrophies. Compr. Physiol. 2018, 8, 1313–1356. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P. The discovery of dystrophin, the protein product of the Duchenne muscular dystrophy gene. FEBS J. 2020, 287, 3879–3887. [Google Scholar] [CrossRef]
- Michele, D.E.; Campbell, K.P. Dystrophin-glycoprotein complex: Post-translational processing and dystroglycan function. J. Biol. Chem. 2003, 278, 15457–15460. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Ohlendieck, K. The biochemical and mass spectrometric profiling of the dystrophin complexome from skeletal muscle. Comput. Struct. Biotechnol. J. 2016, 14, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Spendiff, S.; Roos, A.; Bourque, P.R.; Warman Chardon, J.; Kirschner, J.; Horvath, R.; Lochmuller, H. Advances in the diagnosis of inherited neuromuscular diseases and implications for therapy development. Lancet. Neurol. 2020, 19, 522–532. [Google Scholar] [CrossRef]
- Zweyer, M.; Sabir, H.; Dowling, P.; Gargan, S.; Murphy, S.; Swandulla, D.; Ohlendieck, K. Histopathology of Duchenne muscular dystrophy in correlation with changes in proteomic biomarkers. Histol. Histopathol. 2022, 37, 101–116. [Google Scholar] [CrossRef]
- Ohlendieck, K.; Swandulla, D. Complexity of skeletal muscle degeneration: Multi-systems pathophysiology and organ crosstalk in dystrophinopathy. Pflug. Arch. Eur. J. Physiol. 2021, 473, 1813–1839. [Google Scholar] [CrossRef]
- Meyers, T.A.; Townsend, D. Cardiac Pathophysiology and the Future of Cardiac Therapies in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2019, 20, 4098. [Google Scholar] [CrossRef]
- Chen, Y.W.; Nagaraju, K.; Bakay, M.; McIntyre, O.; Rawat, R.; Shi, R.; Hoffman, E.P. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 2005, 65, 826–834. [Google Scholar] [CrossRef]
- Cruz-Guzman Odel, R.; Rodriguez-Cruz, M.; Escobar Cedillo, R.E. Systemic Inflammation in Duchenne Muscular Dystrophy: Association with Muscle Function and Nutritional Status. BioMed Res. Int. 2015, 2015, 891972. [Google Scholar] [CrossRef]
- De Paepe, B.; De Bleecker, J.L. Cytokines and chemokines as regulators of skeletal muscle inflammation: Presenting the case of Duchenne muscular dystrophy. Mediat. Inflamm. 2013, 2013, 540370. [Google Scholar] [CrossRef]
- Reid, A.L.; Alexander, M.S. The Interplay of Mitophagy and Inflammation in Duchenne Muscular Dystrophy. Life 2021, 11, 648. [Google Scholar] [CrossRef]
- Babaeijandaghi, F.; Cheng, R.; Kajabadi, N.; Soliman, H.; Chang, C.K.; Smandych, J.; Tung, L.W.; Long, R.; Ghassemi, A.; Rossi, F.M.V. Metabolic reprogramming of skeletal muscle by resident macrophages points to CSF1R inhibitors as muscular dystrophy therapeutics. Sci. Transl. Med. 2022, 14, eabg7504. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Argenziano, M.; Tortora, C.; Bellini, G.; Di Paola, A.; Punzo, F.; Rossi, F. Correction: Argenziano, M.; et al. The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases. Int. J. Mol. Sci. 2020, 20, 5875. [Google Scholar] [CrossRef]
- Di Paola, A.; Palumbo, G.; Merli, P.; Argenziano, M.; Tortora, C.; Strocchio, L.; Roberti, D.; Santoro, C.; Perrotta, S.; Rossi, F. Effects of Eltrombopag on In Vitro Macrophage Polarization in Pediatric Immune Thrombocytopenia. Int. J. Mol. Sci. 2020, 22, 97. [Google Scholar] [CrossRef] [PubMed]
- Tortora, C.; Di Paola, A.; Argenziano, M.; Creoli, M.; Marrapodi, M.M.; Cenni, S.; Tolone, C.; Rossi, F.; Strisciuglio, C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines 2022, 10, 847. [Google Scholar] [CrossRef]
- Preteroti, M.W.; Traboulsi, H.; Greiss, P.; Lapohos, O.; Fonseca, G.J.; Eidelman, D.H.; Baglole, C.J. Receptor-mediated effects of Δ9-THC & CBD on the inflammatory response of alveolar macrophages. Immunol. Cell Biol. 2022, 101, 156–170. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Argenziano, M.; Di Paola, A.; Punzo, F. Cannabinoid Receptor Type 2: A Possible Target in SARS-CoV-2 (CoV-19) Infection? Int. J. Mol. Sci. 2020, 21, 3809. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Argenziano, M.; Tortora, C.; Di Paola, A.; Mutarelli, M.; Pota, E.; Di Martino, M.; Di Pinto, D.; Marrapodi, M.M.; Roberti, D.; et al. Effect of CB2 Stimulation on Gene Expression in Pediatric B-Acute Lymphoblastic Leukemia: New Possible Targets. Int. J. Mol. Sci. 2022, 23, 8651. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Manzo, I.; Tortora, C.; Pota, E.; Angelo, V.; Bellini, G.; Di Paola, A.; Verace, F.; Casale, F.; Rossi, F. Effects of CB2 and TRPV1 receptors’ stimulation in pediatric acute T-lymphoblastic leukemia. Oncotarget 2018, 9, 21244–21258. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Tortora, C.; Di Pinto, D.; Manzo, I.; Bellini, G.; Casale, F.; Rossi, F. Anti-proliferative, pro-apoptotic and anti-invasive effect of EC/EV system in human osteosarcoma. Oncotarget 2017, 8, 54459–54471. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Di Martino, M.; Di Paola, A.; Di Pinto, D.; Marrapodi, M.M.; Argenziano, M.; Pota, E. Alteration of osteoclast activity in childhood cancer survivors: Role of iron and of CB2/TRPV1 receptors. PLoS ONE 2022, 17, e0271730. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Palumbo, G.; Punzo, F.; Argenziano, M.; Casale, M.; Di Paola, A.; Locatelli, F.; Perrotta, S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. Int. J. Mol. Sci. 2019, 20, 1049. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 1919. [Google Scholar] [CrossRef]
- Tortora, C.; Di Paola, A.; Creoli, M.; Argenziano, M.; Martinelli, M.; Miele, E.; Rossi, F.; Strisciuglio, C. Effects of CB2 and TRPV1 Stimulation on Osteoclast Overactivity Induced by Iron in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2022, 28, 1244–1253. [Google Scholar] [CrossRef]
- Tortora, C.; Punzo, F.; Argenziano, M.; Di Paola, A.; Tolone, C.; Strisciuglio, C.; Rossi, F. The Role of Cannabinoid Receptor Type 2 in the Bone Loss Associated With Pediatric Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 633–640. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Andrews, N.C.; Schmidt, P.J. Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef]
- Punzo, F.; Tortora, C.; Argenziano, M.; Casale, M.; Perrotta, S.; Rossi, F. Iron chelating properties of Eltrombopag: Investigating its role in thalassemia-induced osteoporosis. PLoS ONE 2018, 13, e0208102. [Google Scholar] [CrossRef]
- Argenziano, M.; Di Paola, A.; Tortora, C.; Di Pinto, D.; Pota, E.; Di Martino, M.; Perrotta, S.; Rossi, F.; Punzo, F. Effects of Iron Chelation in Osteosarcoma. Curr. Cancer Drug Targets 2021, 21, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Tortora, C.; Paola, A.D.; Pota, E.; Martino, M.D.; Pinto, D.D.; Leva, C.D.; Rossi, F. Eltrombopag and its iron chelating properties in pediatric acute myeloid leukemia. Oncotarget 2021, 12, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Palumbo, G.; Tortora, C.; Argenziano, M.; Catanoso, M.; Di Leva, C.; Ceglie, G.; Perrotta, S.; Locatelli, F.; Rossi, F. Eltrombopag in paediatric immune thrombocytopenia: Iron metabolism modulation in mesenchymal stromal cells. Br. J. Haematol. 2022, 197, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.M.; Rossi, F. Emerging Roles of the Iron Chelators in Inflammation. Int. J. Mol. Sci. 2022, 23, 7977. [Google Scholar] [CrossRef]
- Hoffman, E.P. Pharmacotherapy of Duchenne Muscular Dystrophy. Handb. Exp. Pharmacol. 2020, 261, 25–37. [Google Scholar] [CrossRef]
- Manzur, A.Y.; Kuntzer, T.; Pike, M.; Swan, A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2008, CD003725. [Google Scholar] [CrossRef]
- Atilano-Miguel, S.; Barbosa-Cortes, L.; Ortiz-Muniz, R. Duchenne muscular dystrophy: RANK/RANKL/OPG (receptor activator of nuclear factor-kB/RANK ligand/osteoprotegerin) system and glucocorticoids. Bol. Med. Hosp. Infant Mex. 2022, 79, 275–283. [Google Scholar] [CrossRef]
- Howard, Z.M.; Gomatam, C.K.; Rabolli, C.P.; Lowe, J.; Piepho, A.B.; Bansal, S.S.; Accornero, F.; Rafael-Fortney, J.A. Mineralocorticoid receptor antagonists and glucocorticoids differentially affect skeletal muscle inflammation and pathology in muscular dystrophy. JCI Insight 2022, 7, e159875. [Google Scholar] [CrossRef]
- Quattrocelli, M.; Zelikovich, A.S.; Salamone, I.M.; Fischer, J.A.; McNally, E.M. Mechanisms and Clinical Applications of Glucocorticoid Steroids in Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, 39–52. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Adams, J.E.; Padidela, R.; Shaw, N.J.; Hogler, W.; Roper, H.; Hughes, I.; Daniel, A.; Mughal, M.Z. Growth, bone health & ambulatory status of boys with DMD treated with daily vs. intermittent oral glucocorticoid regimen. Bone 2018, 116, 181–186. [Google Scholar] [CrossRef]
- Ricotti, V.; Ridout, D.A.; Scott, E.; Quinlivan, R.; Robb, S.A.; Manzur, A.Y.; Muntoni, F.; NorthStar Clinical, N. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 2013, 84, 698–705. [Google Scholar] [CrossRef]
- Mackenzie, S.J.; Nicolau, S.; Connolly, A.M.; Mendell, J.R. Therapeutic Approaches for Duchenne Muscular Dystrophy: Old and New. Semin. Pediatr. Neurol. 2021, 37, 100877. [Google Scholar] [CrossRef]
- Stephenson, A.A.; Flanigan, K.M. Gene editing and modulation for Duchenne muscular dystrophy. Prog. Mol. Biol. Transl. Sci. 2021, 182, 225–255. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chen, Z.; Yu, Y.; Zhang, N.; Jiang, H.; Zhang, G.; Zhang, Z.; Zhang, B. Current Pharmacological Strategies for Duchenne Muscular Dystrophy. Front. Cell Dev. Biol. 2021, 9, 689533. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Gorvel, L.; Olive, D. Tumor associated macrophage in HPV(+) tumors: Between immunosuppression and inflammation. Semin. Immunol. 2022, 65, 101671. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Bellini, G.; Tortora, C.; Pinto, D.D.; Argenziano, M.; Pota, E.; Paola, A.D.; Martino, M.D.; Rossi, F. Mifamurtide and TAM-like macrophages: Effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget 2020, 11, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Molaaghaee-Rouzbahani, S.; Asri, N.; Jahani-Sherafat, S.; Amani, D.; Masotti, A.; Baghaei, K.; Yadegar, A.; Mirjalali, H.; Rostami-Nejad, M. The modulation of macrophage subsets in celiac disease pathogenesis. Immun. Inflamm. Dis. 2022, 10, e741. [Google Scholar] [CrossRef]
- Fraher, D.; Mann, R.J.; Dubuisson, M.J.; Ellis, M.K.; Yu, T.; Walder, K.; Ward, A.C.; Winkler, C.; Gibert, Y. The endocannabinoid system and retinoic acid signaling combine to influence bone growth. Mol. Cell. Endocrinol. 2021, 529, 111267. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, X.; Geng, D.; Pan, W.; Li, Z.; Wang, G.; Li, D.; Li, C.; Feng, S.; Zhu, L.; et al. H(2)O(2)/NIR-sensitive “two-step” nano theranostic system based hollow mesoporous copper sulfide/hyaluronic acid/JWH133 as an optimally designed delivery system for multidimensional treatment of RA. Int. J. Biol. Macromol. 2022, 225, 298–309. [Google Scholar] [CrossRef]
- Gonzalez-Naranjo, P.; Perez, C.; Giron, R.; Sanchez-Robles, E.M.; Martin-Fontelles, M.I.; Carrillo-Lopez, N.; Martin-Virgala, J.; Naves, M.; Campillo, N.E.; Paez, J.A. New cannabinoid receptor antagonists as pharmacological tool. Bioorganic Med. Chem. 2020, 28, 115672. [Google Scholar] [CrossRef]
- Riddell, D.O.; Hildyard, J.C.W.; Harron, R.C.M.; Hornby, N.L.; Wells, D.J.; Piercy, R.J. Serum inflammatory cytokines as disease biomarkers in the DE50-MD dog model of Duchenne muscular dystrophy. Dis. Model. Mech. 2022, 15, dmm049394. [Google Scholar] [CrossRef]
- Yadav, B.K.; Shah, A.K.; Karunanand, B.; Sudan, D.S.; Sharma, M. Comparative evaluation of INF-gamma as an immunological healing marker based on anti-tubercular treatment among diabetic and non-diabetic pulmonary tuberculosis patients. Horm. Mol. Biol. Clin. Investig. 2022. [Google Scholar] [CrossRef]
- Cai, Z.H.; Tian, Y.G.; Li, J.Z.; Zhao, P.; Li, J.S.; Mei, X.; Bai, Y.P. Peimine ameliorates pulmonary fibrosis via the inhibition of M2-type macrophage polarization through the suppression of P38/Akt/STAT6 signals. Biosci. Rep. 2022, 42, BSR20220986. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Locati, M.; Gammella, E.; Invernizzi, P.; Cairo, G. Iron levels in polarized macrophages: Regulation of immunity and autoimmunity. Autoimmun. Rev. 2012, 11, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jin, S.; Fan, X.; Cao, K.; Liu, Y.; Wang, X.; Ma, Y.; Xiang, L. Cannabidiol Inhibits Inflammation Induced by Cutibacterium acnes-Derived Extracellular Vesicles via Activation of CB2 Receptor in Keratinocytes. J. Inflamm. Res. 2022, 15, 4573–4583. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allara, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Torella, M.; Tortora, C.; Manzo, I.; Giordano, C.; Guida, F.; Luongo, L.; Papale, F.; Rosso, F.; et al. The genetic ablation or pharmacological inhibition of TRPV1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice. Brit. J. Pharm. 2014, 171, 2621–2630. [Google Scholar] [CrossRef] [Green Version]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clements, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argenziano, M.; Pota, V.; Di Paola, A.; Tortora, C.; Marrapodi, M.M.; Giliberti, G.; Roberti, D.; Pace, M.C.; Rossi, F. CB2 Receptor as Emerging Anti-Inflammatory Target in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2023, 24, 3345. https://doi.org/10.3390/ijms24043345
Argenziano M, Pota V, Di Paola A, Tortora C, Marrapodi MM, Giliberti G, Roberti D, Pace MC, Rossi F. CB2 Receptor as Emerging Anti-Inflammatory Target in Duchenne Muscular Dystrophy. International Journal of Molecular Sciences. 2023; 24(4):3345. https://doi.org/10.3390/ijms24043345
Chicago/Turabian StyleArgenziano, Maura, Vincenzo Pota, Alessandra Di Paola, Chiara Tortora, Maria Maddalena Marrapodi, Giulia Giliberti, Domenico Roberti, Maria Caterina Pace, and Francesca Rossi. 2023. "CB2 Receptor as Emerging Anti-Inflammatory Target in Duchenne Muscular Dystrophy" International Journal of Molecular Sciences 24, no. 4: 3345. https://doi.org/10.3390/ijms24043345
APA StyleArgenziano, M., Pota, V., Di Paola, A., Tortora, C., Marrapodi, M. M., Giliberti, G., Roberti, D., Pace, M. C., & Rossi, F. (2023). CB2 Receptor as Emerging Anti-Inflammatory Target in Duchenne Muscular Dystrophy. International Journal of Molecular Sciences, 24(4), 3345. https://doi.org/10.3390/ijms24043345