Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity
Abstract
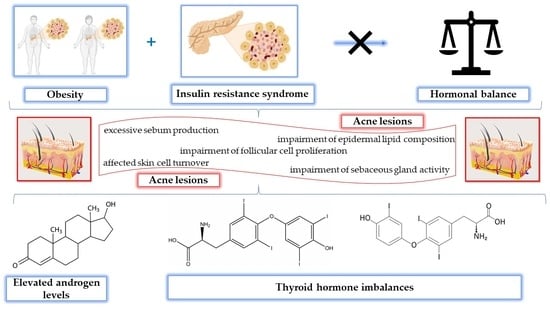
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Methods
- Normal thyroid function (TSH = 0.4–4.0 μIU/mL for adults and 0.39–4.0 μUI/mL for women aged between 13 and 20 years);
- Hypofunction (TSH > 4.0 μUI/mL, regardless of sex or age); and
- Hyperfunction (TSH < 0.39 μUI/mL for women aged between 13 and 20 years, or <0.40 μUI/mL for both women/men >20 years old) (Table 7).
- -
- Patients scoring 1 or 2 were classified as having “mild acne”, having very few papules and a few sporadic comedones, either closed or open, and simple to identify; the affected area of the face being less than half.
- -
- Patients scoring 3 were classified as having “moderate acne”. This type of acne is characterized by numerous comedones, both closed and open, papules, and pustules, and affects more than half of the face. Or there could be just one nodule, inflammatory and non-inflammatory lesions.
- -
- Patients scoring 4 or 5 had “severe acne”. In these cases, the entire face is affected, with numerous papules and pustules, uncommon nodules, and open or closed comedones. Severe inflammatory injuries throughout the face accompanied by nodules and lesions are also present in these patients.
4.2. Statistical Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, W.D. Acne. N. Engl. J. Med. 2005, 352, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, W.J.; Gould, D.J. Prevalence of Facial Acne Vulgaris in Late Adolescence and in Adults. Br. Med. J. 1979, 1, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Goulden, V.; Clark, S.M.; Cunliffe, W.J. Post-Adolescent Acne: A Review of Clinical Features. Br. J. Dermatol. 1997, 136, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Shalita, A.R. Acne Revisited. Arch. Dermatol. 1994, 130, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Kazandjieva, J.; Tsankov, N. Drug-Induced Acne. Clin. Dermatol. 2017, 35, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.J.; Latief, I.; Hassan, I. Update on Etiopathogenesis and Treatment of Acne. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.S.; Krowchuk, D.P.; Leyden, J.J.; Lucky, A.W.; Shalita, A.R.; Siegfried, E.C.; Thiboutot, D.M.; Van Voorhees, A.S.; Beutner, K.A.; Sieck, C.K.; et al. Guidelines of Care for Acne Vulgaris Management. J. Am. Acad. Dermatol. 2007, 56, 651–663. [Google Scholar] [CrossRef]
- Varadi, D.P.; Saqueton, A.C. Perifollicular Elastolysis. Br. J. Dermatol. 1970, 83, 143–150. [Google Scholar] [CrossRef]
- Çerman, A.A.; Aktaş, E.; Altunay, İ.K.; Arıcı, J.E.; Tulunay, A.; Ozturk, F.Y. Dietary Glycemic Factors, Insulin Resistance, and Adiponectin Levels in Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 75, 155–162. [Google Scholar] [CrossRef]
- Emiroğlu, N.; Cengiz, F.P.; Kemeriz, F. Insulin Resistance in Severe Acne Vulgaris. Postep. Dermatol. I Alergol. 2015, 32, 281–285. [Google Scholar] [CrossRef]
- Stefanadi, E.C.; Dimitrakakis, G.; Antoniou, C.-K.; Challoumas, D.; Punjabi, N.; Dimitrakaki, I.A.; Punjabi, S.; Stefanadis, C.I. Metabolic Syndrome and the Skin: A More than Superficial Association. Reviewing the Association between Skin Diseases and Metabolic Syndrome and a Clinical Decision Algorithm for high Risk Patients. Diabetol. Metab. Syndr. 2018, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.F.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic Syndrome: Definitions and Controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Padhi, T. Garima Metabolic Syndrome and Skin: Psoriasis and Beyond. Indian J. Dermatol. 2013, 58, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Megna, M.; Monfrecola, G. Insulin Resistance and Skin Diseases. Sci. World J. 2015, 2015, 479354. [Google Scholar] [CrossRef]
- Melnik, B.C. Acne Vulgaris: The Metabolic Syndrome of the Pilosebaceous Follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef]
- Niepomniszcze, H.; Amad, R.H. Skin Disorders and Thyroid Diseases. J. Endocrinol. Investig. 2001, 24, 628–638. [Google Scholar] [CrossRef]
- Vergou, T.; Mantzou, E.; Tseke, P.; Moustou, A.E.; Katsambas, A.; Alevizaki, M.; Antoniou, C. Association of Thyroid Autoimmunity with Acne in Adult Women. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 413–416. [Google Scholar] [CrossRef]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β Drives Inflammatory Responses to Propionibacterium Acnes in Vitro and in Vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef]
- Heng, A.H.S.; Chew, F.T. Systematic Review of the Epidemiology of Acne Vulgaris. Sci. Rep. 2020, 10, 5754. [Google Scholar] [CrossRef]
- Bungau, S.G.; Tit, D.M.; Vesa, C.M.; Abid, A.; Szilagyi, D.V.; Radu, A.F.; Bungau, A.F.; Tarce, A.G.; Behl, T.; Stoicescu, M.; et al. Non-Conventional Therapeutical Approaches to Acne Vulgaris Related to Its Association with Metabolic Disorders. Eur. J. Pharmacol. 2022, 923, 174936. [Google Scholar] [CrossRef] [PubMed]
- Stathakis, V.; Kilkenny, M.; Marks, R. Descriptive Epidemiology of Acne Vulgaris in the Community. Australas. J. Dermatol. 1997, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bungau, A.F.; Radu, A.F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Endres, L.M. Oxidative Stress and Metabolic Syndrome in Acne Vulgaris: Pathogenetic Connections and Potential Role of Dietary Supplements and Phytochemicals. Biomed. Pharmacother. 2023, 164, 115003. [Google Scholar] [CrossRef] [PubMed]
- Dumont-Wallon, G.; Dréno, B. Specificity of acne in women older than 25 years. Press. Medicale 2008, 37, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Thiboutot, D.; Layton, A.M.; Berson, D.; Perez, M.; Kang, S. Large-Scale International Study Enhances Understanding of an Emerging Acne Population: Adult Females. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.; Guerra, A. Management of Acne in Women Over 25 Years of Age. Actas Dermosifiliogr. 2009, 100, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Karciauskiene, J.; Valiukeviciene, S.; Gollnick, H.; Stang, A. The Prevalence and Risk Factors of Adolescent Acne among Schoolchildren in Lithuania: A Cross-Sectional Study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 733–740. [Google Scholar] [CrossRef]
- El-Akawi, Z.; Abdel-Latif, N.; Abdul-Razzak, K.; Al-Aboosi, M. The Relationship between Blood Lipids Profile and Acne. J. Health Sci. 2007, 53, 596–599. [Google Scholar] [CrossRef]
- Endres, L.; Tit, D.M.; Bungau, S.; Pascalau, N.A.; Maghiar Țodan, L.; Bimbo-Szuhai, E.; Iancu, G.M.; Negrut, N. Incidence and Clinical Implications of Autoimmune Thyroiditis in the Development of Acne in Young Patients. Diagnostics 2021, 11, 794. [Google Scholar] [CrossRef]
- Collier, C.N.; Harper, J.C.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The Prevalence of Acne in Adults 20 Years and Older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef]
- Alshammrie, F.F.; Alshammari, R.; Alharbi, R.M.; Khan, F.H.; Alshammari, S.K. Epidemiology of Acne Vulgaris and Its Association with Lifestyle Among Adolescents and Young Adults in Hail, Kingdom of Saudi Arabia: A Community-Based Study. Cureus 2020, 12, e9277. [Google Scholar] [CrossRef] [PubMed]
- Rathwa, N.; Patel, R.; Palit, S.P.; Ramachandran, A.V.; Begum, R. Genetic Variants of Resistin and Its Plasma Levels: Association with Obesity and Dyslipidemia Related to Type 2 Diabetes Susceptibility. Genomics 2019, 111, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Bar-Tana, J. Type 2 Diabetes—Unmet Need, Unresolved Pathogenesis, MTORC1-Centric Paradigm. Rev. Endocr. Metab. Disord. 2020, 21, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Akhaphong, B.; Baumann, D.C.; Beetch, M.; Lockridge, A.D.; Jo, S.; Wong, A.; Zemanovic, T.; Mohan, R.; Fondevilla, D.L.; Sia, M.; et al. Placental MTOR Complex 1 Regulates Fetal Programming of Obesity and Insulin Resistance in Mice. JCI Insight 2021, 6, e149271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, M.; Wu, S.; Zheng, H. Acne Comorbidities. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kwon, H.H.; Min, S.; Yoon, J.Y.; Suh, D.H. Epidemiology and Risk Factors of Childhood Acne in Korea: A Cross-Sectional Community Based Study. Clin. Exp. Dermatol. 2015, 40, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Hirt, P.A.; Castillo, D.E.; Yosipovitch, G.; Keri, J.E. Skin Changes in the Obese Patient. J. Am. Acad. Dermatol. 2019, 81, 1037–1057. [Google Scholar] [CrossRef]
- Podder, I.; Agarwal, K.; Anurag, A. Metabolic Status, Obesity, and Quality of Life in Patients with Acne Vulgaris: A Cross-Sectional Case-Control Study. Indian J. Dermatol. 2021, 66, 223. [Google Scholar] [CrossRef]
- Nagpal, M.; De, D.; Handa, S.; Pal, A.; Sachdeva, N. Insulin Resistance and Metabolic Syndrome in Young Men with Acne. JAMA Dermatol. 2016, 152, 399–404. [Google Scholar] [CrossRef]
- Kartal, D.; Yildiz, H.; Ertas, R.; Borlu, M.; Utas, S. Association between Isolated Female Acne and Insulin Resistance: A Prospective Study. G. Ital. Dermatol. Venereol. 2016, 151, 353–357. [Google Scholar]
- Shrestha, S. Correlation of Hormonal Profile and Lipid Levels with Female Adult Acne in a Tertiary Care Center of Nepal. J. Nepal Health Res. Counc. 2018, 16, 222–227. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, M.G.; Batista, A.L.F.; Macedo, M.S.; Machado Filho, C.D.S.; Fonseca, F.L.A. Study of Lipid Profile in Adult Women with Acne. Clin. Cosmet. Investig. Dermatol. 2015, 8, 449–454. [Google Scholar] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; Ekiz, O.; Ozuguz, P.; Ustun, I.; Karaca, S.; Dogruk Kacar, S.; Eksioglu, M. Insulin Resistance in Patients with Post-Adolescent Acne. Int. J. Dermatol. 2015, 54, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Shuster, S.; Thody, A.J. The Control and Measurement of Sebum Secretion. J. Investig. Dermatol. 1974, 62, 172–190. [Google Scholar] [CrossRef]
- Ebling, F.J.; Ebling, E.; Skinner, J. The Effects of Thyrotrophic Hormone and of Thyroxine on the Response of the Sebaceous Glands of the Rat to Testosterone. J. Endocrinol. 1970, 48, 83–90. [Google Scholar] [CrossRef]
- Ekiz, O.; Balta, I.; Unlu, E.; Bulbul Sen, B.; Rifaioğlu, E.N.; Dogramaci, A.C. Assessment of Thyroid Function and Lipid Profile in Patients with Postadolescent Acne in a Mediterranean Population from Turkey. Int. J. Dermatol. 2015, 54, 1376–1381. [Google Scholar] [CrossRef]
- Faleri, S.; Feichtner, K.; Ruzicka, T. Severe acne in autoinflammatory diseases. Hautarzt 2016, 67, 897–901. [Google Scholar] [CrossRef]
- Nuzzo, V.; Tauchmanova, L.; Colasanti, P.; Zuccoli, A.; Colao, A. Idiopathic Chronic Urticaria and Thyroid Autoimmunity: Experience of a Single Center. Dermatoendocrinology 2011, 3, 255–258. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Tomé, M.A.; Botana, M.A.; Cadarso-Suárez, C.; Rego-Iraeta, A.; Fernández-Mariño, A.; Mato, J.A.; Solache, I.; Perez-Fernandez, R. Prevalence of Metabolic Syndrome in Galicia (NW Spain) on Four Alternative Definitions and Association with Insulin Resistance. J. Endocrinol. Investig. 2009, 32, 505–511. [Google Scholar]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, E1082–E1143. [Google Scholar] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy Percentage Body Fat Ranges: An Approach for Developing Guidelines Based on Body Mass Index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-Thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial HypertensionThe Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Dréno, B.; Poli, F.; Pawin, H.; Beylot, C.; Faure, M.; Chivot, M.; Auffret, N.; Moyse, D.; Ballanger, F.; Revuz, J. Development and Evaluation of a Global Acne Severity Scale (GEA Scale) Suitable for France and Europe. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 43–48. [Google Scholar] [CrossRef]
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences, 7th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1999. [Google Scholar]







| Characteristics | Total (N = 389) | MPC (N = 163) | EPG (N = 162) | CG (N = 89) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Gender | ||||||||
| Female | 331 | 85.1 | 120 | 73.62 | 155 | 95.68 | 82 | 92.13 |
| Male | 58 | 14.9 | 43 | 26.38 | 7 | 4.32 | 7 | 7.87 |
| Environment | ||||||||
| Rural | 83 | 21.3 | 37 | 22.69 | 30 | 18.52 | 27 | 30.34 |
| Urban | 306 | 78.7 | 126 | 77.31 | 132 | 81.48 | 62 | 69.66 |
| Age | ||||||||
| 12–25 | 93 | 23.9 | 69 | 42.3 | 5 | 3.1 | 19 | 21.3 |
| >25 | 296 | 76.1 | 94 | 57.7 | 157 | 96.9 | 70 | 78.7 |
| Mean age (years) | 28.3 | 25.4 | 33.9 | 26.0 | ||||
| Acne stage | ||||||||
| Light | 89 | 22.88 | 4 | 2.45 | 5 | 3.09 | 80 | 89.89 |
| Moderate | 155 | 39.84 | 92 | 56.44 | 58 | 35.80 | 5 | 5.61 |
| Severe | 145 | 37.28 | 67 | 41.10 | 99 | 61.11 | 4 | 4.89 |
| Concomitant diseases | ||||||||
| Diabetes | 2 | 0.51 | 2 | 1.22 | 2 | 1.23 | - | - |
| High glucose | 4 | 1.03 | 4 | 2.45 | 4 | 2.46 | - | - |
| Insulin resistance syndrome | 104 | 26.73 | 104 | 63.80 | 11 | 6.79 | - | - |
| Overweight | 10 | 2.57 | 10 | 6.13 | 6 | 3.70 | - | - |
| Obesity | 94 | 24.16 | 83 | 50.92 | 11 | 6.79 | - | - |
| Dyslipidemia | 13 | 3.34 | 7 | 4.29 | 6 | 3.70 | - | - |
| High blood pressure | 6 | 1.54 | 3 | 1.84 | 3 | 1.85 | - | - |
| Autoimmune thyroiditis | 78 | 20.05 | 19 | 11.66 | 78 | 20.05 | - | - |
| Hypothyroidism | 8 | 2.06 | 4 | 2.45 | 8 | 2.06 | - | - |
| Autoimmune thyroiditis + Hypothyroidism | 68 | 17.48 | 18 | 11.04 | 68 | 41.98 | - | - |
| Hyperthyroidism | - | - | - | - | - | - | - | - |
| Autoimmune thyroiditis + Hyperthyroidism | 9 | 2.31 | 1 | 0.61 | 9 | 5.55 | - | - |
| Statistics | Age—Study Group | Age—Control Group | Age—Metabolic Preconditioning Group | Age—Endocrine Preconditioning Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Mode | 24 | 19 | 28 | 25 | 20 | 28 | 24 | 19 | 16 | 29 | 32 | 27 |
| Median | 25 | 28 | 30 | 25 | 20 | 28 | 28 | 23 | 26 | 27 | 33.5 | 34 |
| Mean | 26.22 | 27.42 | 30.62 | 26.26 | 19.6 | 28.75 | 28.33 | 24.28 | 26.76 | 27 | 33.72 | 34.36 |
| SE | 0.55 | 0.66 | 0.76 | 0.62 | 0.25 | 0.75 | 2.6 | 0.76 | 1.16 | 0.95 | 0.89 | 0.77 |
| SD | 5.27 | 8.29 | 9.1 | 5.53 | 0.55 | 1.5 | 4.51 | 7.38 | 9.51 | 2.12 | 6.84 | 7.74 |
| Sh-Wilk | 0.89 | 0.95 | 0.97 | 0.89 | 0.68 | 0.63 | 0.99 | 0.91 | 0.91 | 0.91 | 0.98 | 0.99 |
| p (Sh-ilk) | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 | 0.001 | 0.048 | <0.001 | <0.001 | 0.046 | 0.045 | 0.036 |
| Range | 21 | 33 | 37 | 21 | 1 | 3 | 9 | 26 | 37 | 5 | 33 | 37 |
| Minimum | 18 | 14 | 15 | 18 | 19 | 28 | 24 | 14 | 15 | 24 | 14 | 15 |
| Maximum | 39 | 47 | 52 | 39 | 20 | 31 | 33 | 40 | 52 | 29 | 47 | 52 |
| Age Split by the Presence/Absence of the Analyzed Variables | Mean Age (Years) | Statistics Mann–Whitney t Test | p Value |
|---|---|---|---|
| Metabolic preconditioning | 25.8/30.4 | 25,461.5 | <0.001 |
| High glucose level | 31.8/28.3 | 601.5 | 0.452 |
| Insulin resistance syndrome | 23.3/30.1 | 22,087.5 | <0.001 |
| High blood pressure | 44.6/28.2 | 84.5 | 0.011 |
| Dyslipidemia | 35.4/28.2 | 790.5 | 0.02 |
| Diabetes | 24.0/28.3 | 491.5 | 0.512 |
| Overweight | 28.3/28.4 | 1812.5 | 0.815 |
| Obesity | 24.3/29.4 | 17,356 | <0.001 |
| Endocrine preconditioning | 33.9/24.3 | 5841.5 | <0.001 |
| Autoimmune thyroiditis | 34/26.9 | 5973 | <0.001 |
| Hypothyroidism | 25.8/28.4 | 1755 | 0.463 |
| Autoimmune thyroiditis + Hypothyroidism | 34.5/26.9 | 5164 | <0.001 |
| Hyperthyroidism | - | - | - |
| Autoimmune thyroiditis + Hyperthyroidism | 38.6/28.1 | 528 | 0.002 |
| Group | Acne Stage No./% | |||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| Control (89 patients) | 80 | 89.89 | 5 | 5.62 | 4 | 4.49 |
| Metabolic preconditioning (163 patients) | 4 | 2.45 | 92 | 56.44 | 67 | 41.1 |
| Glucose | - | - | - | - | 4 | 5.97 |
| Insulin resistance syndrome | - | - | 63 | 68.49 | 41 | 61.19 |
| High blood pressure | - | - | - | - | 3 | 4.49 |
| Dyslipidemia | 1 | 25 | 2 | 2.17 | 5 | 7.46 |
| Diabetes | - | - | - | - | 2 | 2.99 |
| Overweight | 3 | 75 | 4 | 4.35 | 4 | 5.97 |
| Obesity | - | - | 66 | 71.74 | 17 | 25.37 |
| Endocrine preconditioning (162 patients) | 5 | 3.09 | 58 | 35.8 | 99 | 61.11 |
| Autoimmune thyroiditis | 1 | 20 | 26 | 44.83 | 51 | 51.52 |
| Hypothyroidism | 3 | 60 | 5 | 8.62 | - | - |
| Autoimmune thyroiditis + Hypothyroidism | - | - | 24 | 41.38 | 44 | 44.44 |
| Hyperthyroidism | - | - | - | - | - | - |
| Autoimmune thyroiditis + Hyperthyroidism | 1 | 20 | 4 | 6.89 | 4 | 4.04 |
| Acne Stage Split by the Presence/Absence of the Analyzed Variables | Test | p Value | Test 12–25 Years | p Value | Test >25 Years | p Value |
|---|---|---|---|---|---|---|
| Metabolic preconditioning | 84.897 | <0.001 | 51.859 | <0.001 | 78.360 | <0.001 |
| Glucose | 7.109 | 0.029 | - | - | 7.109 | 0.029 |
| Insulin resistance syndrome | 47.161 | <0.001 | 28.331 | <0.001 | 44.770 | <0.001 |
| High blood pressure | 5.318 | 0.07 | - | - | 5.318 | 0.07 |
| Dyslipidemia | 4.338 | 0.114 | - | - | 4.356 | 0.113 |
| Diabetes | 3.536 | 0.171 | 2.347 | 0.309 | 3.536 | 0.171 |
| Overweight | 0.48 | 0.959 | - | - | 0.476 | 0.788 |
| Obesity | 70.958 | <0.001 | 25.818 | <0.001 | 73.064 | <0.001 |
| Endocrine preconditioning | 97.110 | <0.001 | 2.607 | 0.272 | 98.268 | <0.001 |
| Autoimmune thyroiditis | 44.607 | <0.001 | 2.415 | 0.299 | 40.728 | <0.001 |
| Hypothyroidism | 4.645 | 0.098 | 0.971 | 0.615 | 4.617 | 0.099 |
| Autoimmune thyroiditis + Hypothyroidism | 38.377 | <0.001 | - | - | 34.508 | <0.001 |
| Hyperthyroidism | - | - | - | - | - | - |
| Autoimmune thyroiditis + Hyperthyroidism | 0.845 | 0.655 | - | - | 0.869 | 0.647 |
| The Contingency Tables | Stage of Acne | Statistics | |
|---|---|---|---|
| Samples | Moderate | Mild | |
| Metabolic preconditioning | 92 | 4 | |
| Control | 5 | 80 | |
| Metabolic preconditioning | Severe | Mild | |
| 67 | 4 | ||
| Control | 4 | 80 | |
| Endocrine preconditioning | Moderate | Mild | |
| 58 | 5 | ||
| Control | 5 | 80 | |
| Endocrine preconditioning | Severe | Mild | |
| 99 | 5 | ||
| Control | 4 | 80 | |
| Metabolic and endocrine preconditioning | Moderate | Mild | |
| 10 | 1 | ||
| Control | 5 | 80 | |
| Metabolic and endocrine preconditioning | Severe | Mild | |
| 31 | 1 | ||
| Control | 4 | 80 | |
| Criteria by Groups | Reference Ranges | Ref. |
|---|---|---|
| Metabolic preconditioning group (N = 163) | ||
| High glucose level: Basal glycemia | 100–125 mg/dL | [50] |
| Insulin resistance syndrome: HOMA-IR = (insulin (µU/mL) × glucose level (mg/dL))/405. | >2 | [51] |
| High blood pressure | >130/85 mm Hg | [50] |
Dyslipidemia
| >160 mg/dL <40–50 mg/dL >150 mg/dL | [52] [50] [50] |
Diabetes
| >/=126 mg/dL >/=6.5% >/=200 mg/dL | [53] |
| Overweight: Body mass index | 25.00 to 29.99 kg/m2 | [54] |
| Obesity: Body mass index | >30 kg/m2 | [54] |
| Endocrine preconditioning group (N = 162) | ||
Autoimmune thyroiditis *
| anti-TG > 115 (UI/mL) and/or anti-TPO > 35 (UI/mL) | [55] |
| Hyperthyroidism *: thyroid-stimulating hormone (TSH) | <0.39 (μUI/mL), women, aged between 13–20 years <0.40 (μUI/mL), for both women/men, >20 years | [56] |
| Hypothyroidism *: thyroid-stimulating hormone (TSH) | >4.0 (μUI/mL), regardless sex or age | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungau, A.F.; Tit, D.M.; Bungau, S.G.; Vesa, C.M.; Radu, A.-F.; Marin, R.C.; Endres, L.M.; Moleriu, L.-C. Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity. Int. J. Mol. Sci. 2024, 25, 721. https://doi.org/10.3390/ijms25020721
Bungau AF, Tit DM, Bungau SG, Vesa CM, Radu A-F, Marin RC, Endres LM, Moleriu L-C. Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity. International Journal of Molecular Sciences. 2024; 25(2):721. https://doi.org/10.3390/ijms25020721
Chicago/Turabian StyleBungau, Alexa Florina, Delia Mirela Tit, Simona Gabriela Bungau, Cosmin Mihai Vesa, Andrei-Flavius Radu, Ruxandra Cristina Marin, Laura Maria Endres, and Lavinia-Cristina Moleriu. 2024. "Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity" International Journal of Molecular Sciences 25, no. 2: 721. https://doi.org/10.3390/ijms25020721
APA StyleBungau, A. F., Tit, D. M., Bungau, S. G., Vesa, C. M., Radu, A. -F., Marin, R. C., Endres, L. M., & Moleriu, L. -C. (2024). Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity. International Journal of Molecular Sciences, 25(2), 721. https://doi.org/10.3390/ijms25020721