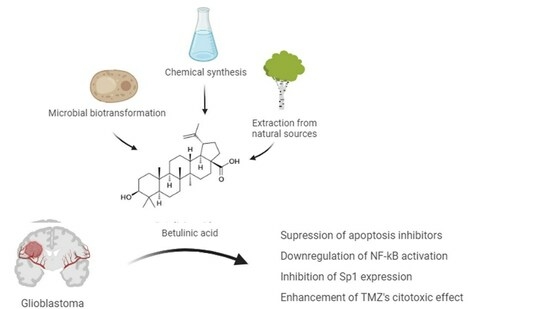
Betulinic Acid for Glioblastoma Treatment: Reality, Challenges and Perspectives
Abstract
:1. Medicine and Plant-Derived Compounds
2. Betulin and Betulinic Acid
Biological Properties of Betulinic Acid
3. Glioblastoma
Glioblastoma’s Invasive Profile
4. Betulinic Acid and Glioblastoma
4.1. Betulinic Acid Effects
4.2. Betulinic Acid Derivative Effects
4.3. Delivery Strategies for Betulinic Acid Application
5. Main Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gershenzon, J.; Ullah, C. Plants protect themselves from herbivores by optimizing the distribution of chemical defenses. Proc. Natl. Acad. Sci. USA 2022, 119, e2120277119. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Akshada Amit, K.; Rajendra Chandrashekar, D.; Chandrakant Shripal, M. Natural Products in Drug Discovery. In Pharmacognosy; Shagufta, P., Areej, A.-T., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 14. [Google Scholar]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.D.; Ma, Q.Q.; Ye, L.; Piao, G.C. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Guo, M.Q.; Sarker, S.D. A review on the latest advances in extraction and analysis of artemisinin. Phytochem. Anal. 2020, 31, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On The Antibacterial Action of Cultures of A Penicillium, With Special Reference To Their Use In The Isolation of B. Influenzae. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New Horizons for Old Drugs and Drug Leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; Int Nat Prod Sci, T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Quiterio, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef]
- Lou, H.H.; Li, H.; Zhang, S.L.; Lu, H.Y.; Chen, Q.H. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Li, C.F.; Zhang, Y.S. Improvement of betulinic acid biosynthesis in yeast employing multiple strategies. BMC Biotechnol. 2016, 16, 59. [Google Scholar] [CrossRef]
- Silva, A.T.; Cerqueira, M.J.; Prudencio, C.; Fernandes, M.H.; Costa-Rodrigues, J.; Teixeira, C.; Gomes, P.; Ferraz, R. Antiproliferative Organic Salts Derived from Betulinic Acid: Disclosure of an Ionic Liquid Selective Against Lung and Liver Cancer Cells. Acs Omega 2019, 4, 5682–5689. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Saneja, A.; Arora, D.; Kumar, R.; Dubey, R.D.; Panda, A.K.; Gupta, P.N. Therapeutic applications of betulinic acid nanoformulations. Ann. N. Y. Acad. Sci. 2018, 1421, 5–18. [Google Scholar] [CrossRef]
- Stork, G.; Uyeo, S.; Wakamatsu, T.; Grieco, P.; Labovitz, J. Total synthesis of lupeol. J. Am. Chem. Soc. 1971, 93, 4945–4947. [Google Scholar] [CrossRef]
- Surendra, K.; Corey, E.J. A Short Enantioselective Total Synthesis of the Fundamental Pentacyclic Triterpene Lupeol. J. Am. Chem. Soc. 2009, 131, 13928–13929. [Google Scholar] [CrossRef]
- Cunha, A.B.; Batista, R.; Castro, M.A.; David, J.M. Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues. Molecules 2021, 26, 1081. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy. Planta Medica 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chen, Z.D.; Nguyen, V.T.; Pezzuto, J.M.; Qiu, S.X.; Lu, Z.Z. A concise semi-synthetic approach to betulinic acid from betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Pezzuto, J.M.; Kim, D.S. Methods of Manufacturing Betulinic Acid. U.S. Patent 5,804,575, 8 September 1998. [Google Scholar]
- Wu, J.N.; Niu, Y.W.; Bakur, A.; Li, H.; Chen, Q.H. Cell-Free Production of Pentacyclic Triterpenoid Compound Betulinic Acid from Betulin by the Engineered Saccharomyces cerevisiae. Molecules 2017, 22, 1075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Shi, X.H.; Wang, J.Q.; Mang, J.; Xu, Z.X. Betulinic Acid Ameliorates Cerebral Injury in Middle Cerebral Artery Occlusion Rats through Regulating Autophagy. ACS Chem. Neurosci. 2021, 12, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chen, H.W.; Zhuang, R.; Zhang, H.J.; Wang, Y.L.; Hu, X.L.; Xu, Y.; Li, J.F.; Li, Y.; Wang, X.Y.; et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Abrishamdar, M.; Sarkaki, A.; Farbood, Y. The effects of betulinic acid chronic administration on the motor, non-motor behaviors, and globus pallidus local field potential power in a rat model of hemiparkinsonism. Iran. J. Basic Med. Sci. 2022, 25, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Navabi, S.P.; Sarkaki, A.; Mansouri, E.; Badavi, M.; Ghadiri, A.; Farbood, Y. The effects of betulinic acid on neurobehavioral activity, electrophysiology and histological changes in an animal model of the Alzheimer’s disease. Behav. Brain Res. 2018, 337, 99–106. [Google Scholar] [CrossRef]
- Li, C.W.; Zhang, C.; Zhou, H.F.; Feng, Y.; Tang, F.; Hoi, M.P.M.; He, C.W.; Ma, D.; Zhao, C.; Lee, S.M.Y. Inhibitory Effects of Betulinic Acid on LPS-Induced Neuroinflammation Involve M2 Microglial Polarization via CaMKK beta-Dependent AMPK Activation. Front. Mol. Neurosci. 2018, 11, 98. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ayeni, G.; Ibeji, C.U.; Olasehinde, T.A.; Chukwuma, C.I.; Koorbanally, N.A.; Islam, M.S. Betulinic Acid Modulates Redox Imbalance and Dysregulated Metabolisms, While Ameliorating Purinergic and Cholinergic Activities in Iron-Induced Neurotoxicity. Rev. Bras. Farmacogn.-Braz. J. Pharmacogn. 2023, 33, 198–207. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Z.H.; Luo, C.X.; Ma, C.Y.; Zhu, L.J.; Kong, L.; Li, R.F.; Wu, J.; Yuan, Z.H.; Yi, J.N. Betulinic acid attenuates cognitive dysfunction, oxidative stress, and inflammation in a model of T-2 toxin-induced brain damage. Environ. Sci. Pollut. Res. 2022, 29, 52098–52110. [Google Scholar] [CrossRef]
- Zhang, Y.; He, N.; Zhou, X.J.; Wang, F.F.; Cai, H.R.; Huang, S.H.; Chen, X.W.; Hu, Z.H.; Jin, X.D. Betulinic acid induces autophagy-dependent apoptosis via Bmi-1/ROS/AMPK-mTOR-ULK1 axis in human bladder cancer cells. Aging-Us 2021, 13, 21251–21267. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, S.Q.; Zheng, Y.F.; Wang, N.; Yang, B.W.; Wang, D.M.; Yang, D.P.; Mei, W.J.; Zhao, Z.M.; Wang, Z.Y. Betulinic acid suppresses breast cancer aerobic glycolysis via caveolin-1/NF-kappa B/c-Myc pathway. Biochem. Pharmacol. 2019, 161, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Liu, P.X.; Wang, N.; Wang, S.Q.; Yang, B.W.; Li, M.; Chen, J.P.; Situ, H.L.; Xie, M.Q.; Lin, Y.; et al. Betulinic Acid Suppresses Breast Cancer Metastasis by Targeting GRP78-Mediated Glycolysis and ER Stress Apoptotic Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 8781690. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.Y.; Liu, C.; Xie, X.Y.; Zhou, J.L. Betulinic acid induces apoptosis and impairs migration and invasion in a mouse model of ovarian cancer. J. Food Biochem. 2020, 44, e13278. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Dong, F.X.; Wang, Y.; Wang, X.A.; Hong, D.F.; Liu, Y.B.; Zhou, J. Betulinic acid induces apoptosis of gallbladder cancer cells via repressing SCD1. Acta Biochim. Et Biophys. Sin. 2020, 52, 200–206. [Google Scholar] [CrossRef]
- Zeng, A.Q.; Hua, H.; Liu, L.; Zhao, J.N. Betulinic acid induces apoptosis and inhibits metastasis of human colorectal cancer cells in vitro and in vivo. Bioorganic Med. Chem. 2019, 27, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Wang, H.X.; Ge, L.; Zhao, Y.Y.; Zhu, K.X.; Chen, Z.S.; Wu, Q.; Xin, Y.; Guo, J.Q. Betulinic acid targets drug-resistant human gastric cancer cells by inducing autophagic cell death, suppresses cell migration and invasion, and modulates the ERK/MEK signaling pathway. Acta Biochim. Pol. 2022, 69, 25–30. [Google Scholar] [CrossRef]
- Jiang, W.K.; Li, X.; Dong, S.; Zhou, W.C. Betulinic acid in the treatment of tumour diseases: Application and research progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef]
- Aswathy, M.; Vijayan, A.; Daimary, U.D.; Girisa, S.; Radhakrishnan, K.V.; Kunnumakkara, A.B. Betulinic acid: A natural promising anticancer drug, current situation, and future perspectives. J. Biochem. Mol. Toxicol. 2022, 36, e23206. [Google Scholar] [CrossRef]
- Atas, M.N.; Ertugrul, B.; Iplik, E.S.; Cakmakoglu, B.; Ergen, A. The inhibitory effect of betulinic acid on epithelial-mesenchymal transition pathway in renal cell carcinoma. Med. Oncol. 2022, 39, 170. [Google Scholar] [CrossRef]
- Seca, A.M.; Pinto, D.C. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Bache, M.; Bernhardt, S.; Passin, S.; Wichmann, H.; Hein, A.; Zschornak, M.; Kappler, M.; Tauber, H.; Paschke, R.; Vordermark, D. Betulinic acid derivatives NVX-207 and B10 for treatment of glioblastoma—An in vitro study of cytotoxicity and radiosensitization. Int. J. Mol. Sci. 2014, 15, 19777–19790. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.; Hsu, T.I.; Yang, W.B.; Kao, T.J.; Wu, M.H.; Huang, Y.N.; Yeh, S.H.; Chuang, J.Y. Betulinic Acid-Mediated Tuning of PERK/CHOP Signaling by Sp1 Inhibition as a Novel Therapeutic Strategy for Glioblastoma. Cancers 2020, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Siminska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.; Taphoorn, M.J.B.; Plaha, P. Advances in the management of glioblastoma. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. Semin. Cancer Biol. 2020, 60, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.C.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Muir, M.; Gopakumar, S.; Traylor, J.; Lee, S.H.; Rao, G.N. Glioblastoma multiforme: Novel therapeutic targets. Expert Opin. Ther. Targets 2020, 24, 605–614. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Tang, Q.; Ren, L.W.; Liu, J.Y.; Li, W.; Fu, W.Q.; Wang, J.H.; Du, G.H. A narrative review of research progress on drug therapies for glioblastoma multiforme. Ann. Transl. Med. 2021, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, M.; De Gregorio, V.; Iorio, A.L.; Giunti, L.; Guidi, M.; de Martino, M.; Genitori, L.; Sardi, I. Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int. J. Mol. Sci. 2018, 19, 2879. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef]
- Steeg, P.S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Khan, I.; Baig, M.H.; Mahfooz, S.; Imran, M.A.; Khan, M.I.; Dong, J.J.; Cho, J.Y.; Hatiboglu, M.A. Nanomedicine for glioblastoma: Progress and future prospects. Semin. Cancer Biol. 2022, 86, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Nakhle, J.; Khattar, K.; Özkan, T.; Boughlita, A.; Abba Moussa, D.; Darlix, A.; Lorcy, F.; Rigau, V.; Bauchet, L.; Gerbal-Chaloin, S.; et al. Mitochondria Transfer from Mesenchymal Stem Cells Confers Chemoresistance to Glioblastoma Stem Cells through Metabolic Rewiring. Cancer Res. Commun. 2023, 3, 1041–1056. [Google Scholar] [CrossRef]
- Chernov, A.N.; Alaverdian, D.A.; Galimova, E.S.; Renieri, A.; Frullanti, E.; Meloni, I.; Shamova, O.V. The phenomenon of multidrug resistance in glioblastomas. Hematol./Oncol. Stem Cell Ther. 2021, 15, 1. [Google Scholar] [CrossRef]
- Kang, H.; Lee, H.; Kim, D.; Kim, B.; Kang, J.; Kim, H.Y.; Youn, H.; Youn, B. Targeting Glioblastoma Stem Cells to Overcome Chemoresistance: An Overview of Current Therapeutic Strategies. Biomedicines 2022, 10, 1308. [Google Scholar] [CrossRef]
- Rocha, J.D.; Uribe, D.; Delgado, J.; Niechi, I.; Alarcon, S.; Erices, J.I.; Melo, R.; Fernandez-Gajardo, R.; Salazar-Onfray, F.; San Martin, R.; et al. A(2B) Adenosine Receptor Enhances Chemoresistance of Glioblastoma Stem-Like Cells under Hypoxia: New Insights into MRP3 Transporter Function. Int. J. Mol. Sci. 2022, 23, 9022. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, K.K.; Wang, J.; Li, X.G.; Zhao, P. Chemoresistance caused by the microenvironment of glioblastoma and the corresponding solutions. Biomed. Pharmacother. 2019, 109, 39–46. [Google Scholar] [CrossRef]
- Anderson, G. Glioblastoma chemoresistance: Roles of the mitochondrial melatonergic pathway. Cancer Drug Resist. 2020, 3, 334–355. [Google Scholar] [CrossRef]
- Erices, J.I.; Bizama, C.; Niechi, I.; Uribe, D.; Rosales, A.; Fabres, K.; Navarro-Martinez, G.; Torres, A.; San Martin, R.; Roa, J.C.; et al. Glioblastoma Microenvironment and Invasiveness: New Insights and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 7047. [Google Scholar] [CrossRef]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 1932. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.; Han, M.; Bjerkvig, R.; Niclou, S.P. Chapter Two—Novel facets of glioma invasion. In International Review of Cell and Molecular Biology; Thomas, C., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 360, pp. 33–64. [Google Scholar]
- Velasquez, C.; Mansouri, S.; Mora, C.; Nassiri, F.; Suppiah, S.; Martino, J.; Zadeh, G.; Fernandez-Luna, J.L. Molecular and Clinical Insights into the Invasive Capacity of Glioblastoma Cells. J. Oncol. 2019, 2019, 1740763. [Google Scholar] [CrossRef]
- Hatoum, A.; Mohammed, R.; Zakieh, O. The unique invasiveness of glioblastoma and possible drug targets on extracellular matrix. Cancer Manag. Res. 2019, 11, 1843–1855. [Google Scholar] [CrossRef]
- Chouleur, T.; Tremblay, M.L.; Bikfalvi, A. Mechanisms of invasion in glioblastoma. Curr. Opin. Oncol. 2020, 32, 631–639. [Google Scholar] [CrossRef]
- Guyon, J.; Fernandez-Moncada, I.; Larrieu, C.M.; Bouchez, C.L.; Zottola, A.C.P.; Galvis, J.; Chouleur, T.; Burban, A.; Joseph, K.; Ravi, V.M.; et al. Lactate dehydrogenases promote glioblastoma growth and invasion via a metabolic symbiosis. EMBO Mol. Med. 2022, 14, e15343. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Du, Q.; Li, L.; Xi, X.; Liu, Q.L.; Li, W.J.; Liu, S.Q. Eriodictyol inhibits glioblastoma migration and invasion by reversing EMT via downregulation of the P38 MAPK/GSK-3 beta/ZEB1 pathway. Eur. J. Pharmacol. 2021, 900, 174069. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.N.; Liu, M.; Zhang, S.S.; Shang, Y.F.; Song, F.H.; Zhang, H.W.; Du, G.H.; Wang, Y.H. Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects. Molecules 2022, 27, 6148. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Ronellenfitsch, M.W.; Thiepold, A.L.; Harter, P.N.; Reichert, S.; Kögel, D.; Paschke, R.; Mittlebron, M.; Weller, M.; Steinbach, J.P.; et al. Hypoxia enhances the antiglioma cytotoxicity of B10, a glycosylated derivative of betulinic acid. PLoS ONE 2014, 9, e94921. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Molina, D.; Mao, X.; Alfonso-Triguero, P.; Lorenzo, J.; Bruna, J.; Yuste, V.J.; Candiota, A.P.; Novio, F. Advances in preclinical/clinical glioblastoma treatment: Can nanoparticles be of help? Cancers 2022, 14, 4960. [Google Scholar] [CrossRef] [PubMed]
- Yaozu, Z.; Liu, Y.; Zhao, H.; Peng, P.; Tingbao, Z.; Jincao, C. Betulinic acid inhibits glioma cell viability by downregulation of NF-κB and enhancement of apoptosis. Trop. J. Pharm. Res. 2020, 19, 2545–2551. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, R.J.; Liu, M.; Feng, J.F.; Chen, J.; Hu, K.L. Remodeling the blood-brain barrier microenvironment by natural products for brain tumor therapy. Acta Pharm. Sin. B 2017, 7, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.X.; Gao, L.; Tan, Y.Q.; Cai, J.Y.; Ye, Z.; Chen, A.T.; Xu, Y.; Zhao, L.Y.; Tong, S.A.; et al. Betulinic acid self-assembled nanoparticles for effective treatment of glioblastoma. J. Nanobiotechnol. 2022, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Betulinic acid inhibits high glucose-induced vascular smooth muscle cells proliferation and migration. J. Cell. Biochem. 2010, 111, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Zha, W.; Zi, J. Biotechnological production of betulinic acid and derivatives and their applications. Appl. Microbiol. Biotechnol. 2020, 104, 3339–3348. [Google Scholar] [CrossRef]
- Lombrea, A.; Scurtu, A.D.; Avram, S.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipins, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer potential of betulonic acid derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Bai, X.; Wang, Y.; Wang, M. Betulinic acid derivative B10 inhibits glioma cell proliferation through suppression of SIRT1, acetylation of FOXO3a and upregulation of Bim/PUMA. Biomed. Pharmacother. 2017, 92, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.; Wacheck, V.; Buckley, J.; Nagy, K.; Thalhammer, J.; Paschke, R.; Triche, T.; Jansen, B.; Selzer, E. Characterization of NVX-207, a novel betulinic acid-derived anti-cancer compound. Eur. J. Clin. Investig. 2009, 39, 384–394. [Google Scholar] [CrossRef]
- Krol, S.K.; Bębenek, E.; Sławińska-Brych, A.; Dmoszyńska-Graniczka, M.; Boryczka, S.; Stepulak, A. Synthetic betulin derivatives inhibit growth of glioma cells in vitro. Anticancer Res. 2020, 40, 6151–6158. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kumar, V.; Srivastava, S.K.; Agarwal, S.K.; Burman, A.C. Betulinic acid derivatives as anticancer agents: Structure activity relationship. Anti-Cancer Agents Med. Chem. 2006, 6, 271–279. [Google Scholar] [CrossRef]
- Csuk, R. Betulinic acid and its derivatives: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 913–923. [Google Scholar] [CrossRef]
- Roque, D.; Cruz, N.; Ferreira, H.A.; Reis, C.P.; Matela, N.; Herculano-Carvalho, M.; Cascão, R.; Faria, C.C. Nanoparticle-Based Treatment in Glioblastoma. J. Pers. Med. 2023, 13, 1328. [Google Scholar] [CrossRef]
- Suresh, C.; Zhao, H.; Gumbs, A.; Chetty, C.S.; Bose, H.S. New ionic derivatives of betulinic acid as highly potent anti-cancer agents. Bioorganic Med. Chem. Lett. 2012, 22, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chrobak, E.; Rzepka, Z.; Wrześniok, D. New Betulin Derivatives with Nitrogen Heterocyclic Moiety—Synthesis and Anticancer Activity In Vitro. Biomolecules 2022, 12, 1540. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Ma, C.; Zhao, H.T.; Zhang, S.Q.; Liu, J.; Liu, F.Y.; Chen, Z.M.; Chen, A.T.; Yang, X.; Avery, J.; et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics 2019, 9, 6991–7002. [Google Scholar] [CrossRef] [PubMed]
- Alshweiat, A.; Jaber, M.; Athamneh, T.; Oqal, M. Recent insights into nanoformulation delivery systems of flavonoids against glioblastoma. J. Drug Deliv. Sci. Technol. 2023, 91, 105271. [Google Scholar] [CrossRef]
- Gusmão, L.A.; Matsuo, F.S.; Barbosa, H.F.G.; Tedesco, A.C. Advances in nano-based materials for glioblastoma multiforme diagnosis: A mini-review. Front. Nanotechnol. 2022, 4, 836802. [Google Scholar] [CrossRef]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020, 11, 5687. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Talaie, Z.; Syahir, A. Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable. Pharmaceutics 2022, 14, 1697. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, S.; Vieira, M.; Prudêncio, C.; Ferraz, R. Betulinic Acid for Glioblastoma Treatment: Reality, Challenges and Perspectives. Int. J. Mol. Sci. 2024, 25, 2108. https://doi.org/10.3390/ijms25042108
Fernandes S, Vieira M, Prudêncio C, Ferraz R. Betulinic Acid for Glioblastoma Treatment: Reality, Challenges and Perspectives. International Journal of Molecular Sciences. 2024; 25(4):2108. https://doi.org/10.3390/ijms25042108
Chicago/Turabian StyleFernandes, Sílvia, Mariana Vieira, Cristina Prudêncio, and Ricardo Ferraz. 2024. "Betulinic Acid for Glioblastoma Treatment: Reality, Challenges and Perspectives" International Journal of Molecular Sciences 25, no. 4: 2108. https://doi.org/10.3390/ijms25042108
APA StyleFernandes, S., Vieira, M., Prudêncio, C., & Ferraz, R. (2024). Betulinic Acid for Glioblastoma Treatment: Reality, Challenges and Perspectives. International Journal of Molecular Sciences, 25(4), 2108. https://doi.org/10.3390/ijms25042108