Structure, Functions, and Implications of Selected Lipocalins in Human Disease
Abstract
:1. Introduction
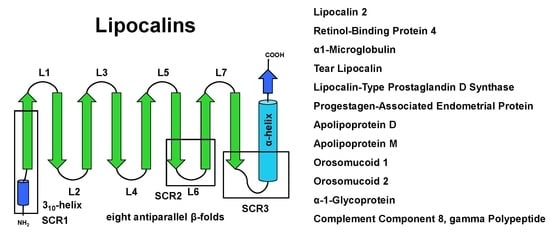
2. Structure and Sequence Properties of Lipocalins
3. Lipocalins of Significance: An Overview
3.1. α1-microglobulin
3.2. Retinol Binding Protein 4
3.3. Lipocalin 2
3.4. Lipocalin-Type PGD2 Synthase Protein
4. Implications of Lipocalins in Diseases
4.1. Lipocalins in Immune Responses
4.2. Lipocalins in the Regulation of Metabolism
4.3. Lipocalins in Aging and Development
4.4. Lipocalins in Reproduction
4.5. Lipocalins in Human Cancer
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A1M | α1-microglobulin |
| AGP | α1-glycoprotein |
| ApoD | Apolipoprotein D |
| ApoM | Apolipoprotein M |
| LCN | Lipocalin |
| L-PGDS | Lipocalin-type prostaglandin D synthase |
| MUP | Major urinary protein |
| RBP4 | Retinol binding protein 4 |
| TLR | Toll-like receptor |
References
- Pervaiz, S.; Brew, K. Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1987, 1, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Charkoftaki, G.; Wang, Y.; McAndrews, M.; Bruford, E.A.; Thompson, D.C.; Vasiliou, V.; Nebert, D.W. Update on the human and mouse lipocalin (LCN) gene family, including evidence the mouse Mup cluster is result of an “evolutionary bloom”. Hum. Genom. 2019, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Wang, X.; Li, S.; Liu, Y.; Akbar, R.; Fan, G.C. Lipocalin family proteins and their diverse roles in cardiovascular disease. Pharmacol. Ther. 2023, 244, 108385. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Åkerström, B.; Sanchez, D. Editorial: Functional profile of the lipocalin protein family. Front. Physiol. 2022, 13, 904702. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological functions of RBP4 and its relevance for human diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef] [PubMed]
- Bergwik, J.; Kristiansson, A.; Allhorn, M.; Gram, M.; Åkerström, B. Structure, functions, and physiological roles of the lipocalin α(1)-microglobulin (A1M). Front. Physiol. 2021, 12, 645650. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M. Into the labyrinth of the lipocalin α1-acid glycoprotein. Front. Physiol. 2021, 12, 686251. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, L. β-lactoglobulin and glycodelin: Two sides of the same coin? Front. Physiol. 2021, 12, 678080. [Google Scholar] [CrossRef]
- Redl, B.; Habeler, M. The diversity of lipocalin receptors. Biochimie 2022, 192, 22–29. [Google Scholar] [CrossRef]
- Wang, J.C.; Ku, H.Y.; Chen, T.S.; Chuang, H.S. Detection of low-abundance biomarker lipocalin 1 for diabetic retinopathy using optoelectrokinetic bead-based immunosensing. Biosens. Bioelectron. 2017, 89 Pt 2, 701–709. [Google Scholar] [CrossRef]
- Karnati, R.; Laurie, D.E.; Laurie, G.W. Lacritin and the tear proteome as natural replacement therapy for dry eye. Exp. Eye Res. 2013, 117, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A master mediator of intestinal and metabolic inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Tegoni, M.; Pelosi, P.; Vincent, F.; Spinelli, S.; Campanacci, V.; Grolli, S.; Ramoni, R.; Cambillau, C. Mammalian odorant binding proteins. Biochim. Biophys. Acta 2000, 1482, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Cederlund, M.; Ghosh, F.; Arnér, K.; Andréasson, S.; Akerström, B. Vitreous levels of oxidative stress biomarkers and the radical-scavenger α1-microglobulin/A1M in human rhegmatogenous retinal detachment. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 725–732. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The role of obesity in type 2 diabetes mellitus—An overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed]
- Serna, M.; Giles, J.L.; Morgan, B.P.; Bubeck, D. Structural basis of complement membrane attack complex formation. Nat. Commun. 2016, 7, 10587. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Pocacqua, V. The acute phase protein alpha1-acid glycoprotein: A model for altered glycosylation during diseases. Curr. Protein Pept. Sci. 2007, 8, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Roth, K.; Kunert-Keil, C.; Scholz, C.; Schulze, S.; Mylonas, I.; Friese, K.; Jeschke, U. Glycodelin protein and mRNA is downregulated in human first trimester abortion and partially upregulated in mole pregnancy. J. Histochem. Cytochem. 2008, 56, 477–485. [Google Scholar] [CrossRef]
- Schneider, M.A.; Granzow, M.; Warth, A.; Schnabel, P.A.; Thomas, M.; Herth, F.J.; Dienemann, H.; Muley, T.; Meister, M. Glycodelin: A new biomarker with immunomodulatory functions in non-small cell lung cancer. Clin. Cancer Res. 2015, 21, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, R. The role of SCAP/SREBP as central regulators of lipid metabolism in hepatic steatosis. Int. J. Mol. Sci. 2024, 25, 1109. [Google Scholar] [CrossRef]
- Grzyb, J.; Latowski, D.; Strzałka, K. Lipocalins—A family portrait. J. Plant Physiol. 2006, 163, 895–915. [Google Scholar] [CrossRef]
- Du, Z.P.; Wu, B.L.; Wu, X.; Lin, X.H.; Qiu, X.Y.; Zhan, X.F.; Wang, S.H.; Shen, J.H.; Zheng, C.P.; Wu, Z.Y.; et al. A systematic analysis of human lipocalin family and its expression in esophageal carcinoma. Sci. Rep. 2015, 5, 12010. [Google Scholar] [CrossRef] [PubMed]
- Yao Mattisson, I.; Christoffersen, C. Apolipoprotein M and its impact on endothelial dysfunction and inflammation in the cardiovascular system. Atherosclerosis 2021, 334, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Z.; Guo, Z.; Zhang, H.; Zhang, Z.; Luo, M.; Hou, H.; Huang, A.; Dong, Y.; Wang, D. The crystal structure of human protein α1M reveals a chromophore-binding site and two putative protein-protein interfaces. Biochem. Biophys. Res. Commun. 2013, 439, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Rutardottir, S.; Nilsson, E.J.; Pallon, J.; Gram, M.; Åkerström, B. The cysteine 34 residue of A1M/α1-microglobulin is essential for protection of irradiated cell cultures and reduction of carbonyl groups. Free Radic. Res. 2013, 47, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, B.; Gram, M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic. Biol. Med. 2014, 74, 274–282. [Google Scholar] [CrossRef]
- Cederlund, M.; Deronic, A.; Pallon, J.; Sørensen, O.E.; Åkerström, B. A1M/α1-microglobulin is proteolytically activated by myeloperoxidase, binds its heme group and inhibits low density lipoprotein oxidation. Front. Physiol. 2015, 6, 11. [Google Scholar] [CrossRef]
- Gunnarsson, R.; Åkerström, B.; Hansson, S.R.; Gram, M. Recombinant alpha-1-microglobulin: A potential treatment for preeclampsia. Drug Discov. Today 2017, 22, 736–743. [Google Scholar] [CrossRef]
- Kristiansson, A.; Gram, M.; Flygare, J.; Hansson, S.R.; Åkerström, B.; Storry, J.R. The role of α1-microglobulin (A1M) in erythropoiesis and erythrocyte homeostasis-therapeutic opportunities in hemolytic conditions. Int. J. Mol. Sci. 2020, 21, 7234. [Google Scholar] [CrossRef]
- Kristiansson, A.; Ahlstedt, J.; Holmqvist, B.; Brinte, A.; Tran, T.A.; Forssell-Aronsson, E.; Strand, S.-E.; Gram, M.; Åkerström, B. Protection of kidney function with human antioxidation protein α1-microglobulin in a mouse 177Lu-DOTATATE radiation therapy model. Antioxid. Redox Signal. 2018, 30, 1746–1759. [Google Scholar] [CrossRef]
- Romantsik, O.; Agyemang, A.A.; Sveinsdóttir, S.; Rutardóttir, S.; Holmqvist, B.; Cinthio, M.; Mörgelin, M.; Gumus, G.; Karlsson, H.; Hansson, S.R.; et al. The heme and radical scavenger α1-microglobulin (A1M) confers early protection of the immature brain following preterm intraventricular hemorrhage. J. Neuroinflam. 2019, 16, 122. [Google Scholar] [CrossRef]
- Erlandsson, L.; Ducat, A.; Castille, J.; Zia, I.; Kalapotharakos, G.; Hedström, E.; Vilotte, J.-L.; Vaiman, D.; Hansson, S.R. Alpha-1 microglobulin as a potential therapeutic candidate for treatment of hypertension and oxidative stress in the STOX1 preeclampsia mouse model. Sci. Rep. 2019, 9, 8561. [Google Scholar] [CrossRef] [PubMed]
- Welles, J.E.; Toro, A.L.; Sunilkumar, S.; Stevens, S.A.; Purnell, C.J.; Kimball, S.R.; Dennis, M.D. Retinol-binding protein 4 mRNA translation in hepatocytes is enhanced by activation of mTORC1. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E306–E315. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. STRA6-catalyzed vitamin A influx, efflux, and exchange. J. Membr. Biol. 2012, 245, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kiernan, U.A.; Shi, L.; Phillips, D.A.; Kahn, B.B.; Hu, F.B.; Manson, J.E.; Albert, C.M.; Rexrode, K.M. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: A prospective analysis among women in the nurses’ health study. Circulation 2013, 127, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shi, D.; Suzuki, T.; Xia, Z.; Zhang, H.; Araki, K.; Wakana, S.; Takeda, N.; Yamamura, K.-I.; Jin, S.; et al. Severe ocular phenotypes in Rbp4-deficient mice in the C57BL/6 genetic background. Lab. Investig. 2016, 96, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Suzuki, T.; Shen, J.; Wakana, S.; Araki, K.; Yamamura, K.-i.; Lei, L.; Li, Z. Rescue of retinal morphology and function in a humanized mouse at the mouse retinol-binding protein locus. Lab. Investig. 2017, 97, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.M.; Nelson, C.; Tarlé, S.A.; Pribila, J.T.; Bardakjian, T.; Woods, S.; Schneider, A.; Glaser, T. Biochemical basis for dominant inheritance, variable penetrance, and maternal effects in RBP4 congenital eye disease. Cell 2015, 161, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Carss, K.; Raymond, F.L.; Islam, F.; Nihr BioResource-Rare Diseases, C.; Moore, A.T.; Michaelides, M.; Arno, G. Vitamin A deficiency due to bi-allelic mutation of RBP4: There’s more to it than meets the eye. Ophthalmic Genet. 2017, 38, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; Jasani, K.M.; Shanks, M.; Clouston, P.; MacLaren, R.E. A novel homozygous c.67C>T variant in retinol binding protein 4 (RBP4) associated with retinitis pigmentosa and childhood acne vulgaris. Ophthalmic Genet. 2020, 41, 288–292. [Google Scholar] [CrossRef]
- Wan, K.; Zhao, J.; Deng, Y.; Chen, X.; Zhang, Q.; Zeng, Z.; Zhang, L.; Chen, Y. A genetic polymorphism in RBP4 is associated with coronary artery disease. Int. J. Mol. Sci. 2014, 15, 22309–22319. [Google Scholar] [CrossRef]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Vieira, P.M.; Yore, M.M.; Sontheimer-Phelps, A.; Castoldi, A.; Norseen, J.; Aryal, P.; Simonyté Sjödin, K.; Kahn, B.B. Retinol binding protein 4 primes the NLRP3 inflammasome by signaling through Toll-like receptors 2 and 4. Proc. Natl. Acad. Sci. USA 2020, 117, 31309–31318. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Yuen, J.J.; Jiang, H.; Kahn, B.B.; Blaner, W.S. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology 2016, 64, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Kulterer, O.C.; Spirk, K.; Mitulović, G.; Marculescu, R.; Bilban, M.; Baumgartner-Parzer, S.; Kautzky-Willer, A.; Kenner, L.; Plutzky, J.; et al. Intact vitamin A transport is critical for cold-mediated adipose tissue browning and thermogenesis. Mol. Metab. 2020, 42, 101088. [Google Scholar] [CrossRef]
- Kraus, B.J.; Sartoretto, J.L.; Polak, P.; Hosooka, T.; Shiroto, T.; Eskurza, I.; Lee, S.A.; Jiang, H.; Michel, T.; Kahn, B.B. Novel role for retinol-binding protein 4 in the regulation of blood pressure. FASEB J. 2015, 29, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Fedders, R.; Muenzner, M.; Schupp, M. Retinol binding protein 4 and its membrane receptors: A metabolic perspective. Horm. Mol. Biol. Clin. Investig. 2015, 22, 27–37. [Google Scholar] [CrossRef]
- Zhao, P.; Elks, C.M.; Stephens, J.M. The induction of lipocalin-2 protein expression in vivo and in vitro. J. Biol. Chem. 2014, 289, 5960–5969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Shan, X.; Syed, F.; Lewandowski, K.C.; O’Hare, J.P.; Randeva, H.S. Ex vivo and in vivo regulation of lipocalin-2, a novel adipokine, by insulin. Diabetes Care 2009, 32, 129–131. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. Cellular and molecular mechanisms of insulin resistance. Curr. Tissue Microenviron. Rep. 2024; in press. [Google Scholar] [CrossRef]
- Li, D.; Yan Sun, W.; Fu, B.; Xu, A.; Wang, Y. Lipocalin-2-The myth of its expression and function. Basic Clin. Pharmacol. Toxicol. 2020, 127, 142–151. [Google Scholar] [CrossRef]
- Chaudhary, N.; Choudhary, B.S.; Shah, S.G.; Khapare, N.; Dwivedi, N.; Gaikwad, A.; Joshi, N.; Raichanna, J.; Basu, S.; Gurjar, M.; et al. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int. J. Cancer 2021, 149, 1495–1511. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Dá Mesquita, S.; Sousa, J.C.; Correia-Neves, M.; Sousa, N.; Palha, J.A.; Marques, F. From the periphery to the brain: Lipocalin-2, a friend or foe? Prog. Neurobiol. 2015, 131, 120–136. [Google Scholar] [CrossRef]
- Courbon, G.; Francis, C.; Gerber, C.; Neuburg, S.; Wang, X.; Lynch, E.; Isakova, T.; Babitt, J.L.; Wolf, M.; Martin, A.; et al. Lipocalin 2 stimulates bone fibroblast growth factor 23 production in chronic kidney disease. Bone Res. 2021, 9, 35. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front. Physiol. 2016, 7, 430. [Google Scholar] [CrossRef]
- Asaf, S.; Maqsood, F.; Jalil, J.; Sarfraz, Z.; Sarfraz, A.; Mustafa, S.; Ojeda, I.C. Lipocalin 2-not only a biomarker: A study of current literature and systematic findings of ongoing clinical trials. Immunol. Res. 2023, 71, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Togawa, A.; Duncan, G.S.; Elia, A.J.; You-Ten, A.; Wakeham, A.; Fong, H.E.; Cheung, C.C.; Mak, T.W. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2006, 103, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.K.; Gasterich, N.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 receptors: Facts, fictions, and myths. Front. Immunol. 2023, 14, 1229885. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.; Borkham-Kamphorst, E.; Henning, M.; Yagmur, E.; Gassler, N.; Liedtke, C.; Berger, T.; Mak, T.W.; Weiskirchen, R. Lipocalin-2 (LCN2) regulates PLIN5 expression and intracellular lipid droplet formation in the liver. Biochim. Biophys. Acta 2014, 1842, 1513–1524. [Google Scholar] [CrossRef]
- Urade, Y. Biochemical and structural characteristics, gene regulation, physiological, pathological and clinical features of lipocalin-type prostaglandin D(2) synthase as a multifunctional lipocalin. Front. Physiol. 2021, 12, 718002. [Google Scholar] [CrossRef]
- Urade, Y.; Eguchi, N.; Hayaishi, O. Lipocalin-type prostaglandin D synthase as an enzymic lipocalin. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2000–2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6621/ (accessed on 6 April 2024).
- Islam, M.A.; Khairnar, R.; Fleishman, J.; Thompson, K.; Kumar, S. Lipocalin-type prostaglandin D2 synthase protein- A central player in metabolism. Pharm. Res. 2022, 39, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, A.; Yamada, M.; Fujimori, K.; Miyamoto, Y.; Kusumoto, T.; Nakajima, H.; Inui, T. Lipocalin-type prostaglandin D synthase protects against oxidative stress-induced neuronal cell death. Biochem. J. 2012, 443, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, S.; Yoshida, T.; Inui, T.; Gohda, K.; Kobayashi, Y.; Fujimori, K.; Tsurumura, T.; Aritake, K.; Urade, Y.; Ohkubo, T. NMR solution structure of lipocalin-type prostaglandin D synthase: Evidence for partial overlapping of catalytic pocket and retinoic acid-binding pocket within the central cavity. J. Biol. Chem. 2007, 282, 31373–31379, Erratum in J. Biol. Chem. 2008, 283, 8772. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Fukuda, T. Prostaglandin D2 and TH2 inflammation in the pathogenesis of bronchial asthma. Korean J. Intern. Med. 2011, 26, 8–18, Erratum in Korean J. Intern. Med. 2011, 26, 253. [Google Scholar] [CrossRef]
- Virtue, S.; Masoodi, M.; Velagapudi, V.; Tan, C.Y.; Dale, M.; Suorti, T.; Slawik, M.; Blount, M.; Burling, K.; Campbell, M.; et al. Lipocalin prostaglandin D synthase and PPARγ2 coordinate to regulate carbohydrate and lipid metabolism in vivo. PLoS ONE 2012, 7, e39512. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Aritake, K.; Oishi, Y.; Nagata, N.; Maehara, T.; Lazarus, M.; Urade, Y. L-PGDS-produced PGD2 in premature, but not in mature, adipocytes increases obesity and insulin resistance. Sci. Rep. 2019, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.; Ganfornina, M.D. The lipocalin apolipoprotein D functional portrait: A systematic review. Front. Physiol. 2021, 12, 738991. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, A.; Lee, W.H.; Suk, K. Lipocalin-2 in diabetic complications of the nervous system: Physiology, pathology, and beyond. Front. Physiol. 2021, 12, 638112. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, B.J. Tear lipocalin and lipocalin-interacting membrane receptor. Front. Physiol. 2021, 12, 684211. [Google Scholar] [CrossRef]
- Stopková, R.; Otčenášková, T.; Matějková, T.; Kuntová, B.; Stopka, P. Biological roles of lipocalins in chemical communication, reproduction, and regulation of microbiota. Front. Physiol. 2021, 12, 740006. [Google Scholar] [CrossRef]
- Frances, L.; Tavernier, G.; Viguerie, N. Adipose-derived lipid-binding proteins: The good, the bad and the metabolic diseases. Int. J. Mol. Sci. 2021, 22, 10460. [Google Scholar] [CrossRef]
- Kim, J.-H.; Han, J.; Suk, K. Protective effects of complement component 8 gamma against blood-brain barrier breakdown. Front. Physiol. 2021, 12, 671250. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Lecchi, C. The immune functions of α1 acid glycoprotein. Curr. Protein Pept. Sci. 2019, 20, 505–524. [Google Scholar] [CrossRef]
- Bergwik, J.; Kristiansson, A.; Welinder, C.; Göransson, O.; Hansson, S.R.; Gram, M.; Erlandsson, L.; Åkerström, B. Knockout of the radical scavenger α1-microglobulin in mice results in defective bikunin synthesis, endoplasmic reticulum stress and increased body weight. Free Radic. Biol. Med. 2021, 162, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Rui, L. Chapter Fifteen—Lipocalin 13 regulation of glucose and lipid metabolism in obesity. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 91, pp. 369–383. [Google Scholar] [CrossRef]
- Gao, R.; Wang, H.; Li, T.; Wang, J.; Ren, Z.; Cai, N.; Ai, H.; Li, S.; Lu, Y.; Zhu, Y.; et al. Secreted MUP1 that reduced under ER stress attenuates ER stress induced insulin resistance through suppressing protein synthesis in hepatocytes. Pharmacol. Res. 2023, 187, 106585. [Google Scholar] [CrossRef]
- Flores-Cortez, Y.A.; Barragán-Bonilla, M.I.; Mendoza-Bello, J.M.; González-Calixto, C.; Flores-Alfaro, E.; Espinoza-Rojo, M. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol. Med. Rep. 2022, 26, 244. [Google Scholar] [CrossRef]
- Ding, B.S.; Yang, D.; Swendeman, S.L.; Christoffersen, C.; Nielsen, L.B.; Friedman, S.L.; Powell, C.A.; Hla, T.; Cao, Z. Aging suppresses sphingosine-1-phosphate chaperone ApoM in circulation resulting in maladaptive organ repair. Dev. Cell 2020, 53, 677–690.e4. [Google Scholar] [CrossRef]
- Dekens, D.W.; Eisel, U.L.M.; Gouweleeuw, L.; Schoemaker, R.G.; De Deyn, P.P.; Naudé, P.J.W. Lipocalin 2 as a link between ageing, risk factor conditions and age-related brain diseases. Ageing Res. Rev. 2021, 70, 101414. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Villar-Piqué, A.; Schmitz, M.; Schmidt, C.; Varges, D.; Goebel, S.; Bunck, T.; Lindemann, H.; Bogner, C.; Santana, I.; et al. Plasma lipocalin 2 in Alzheimer’s disease: Potential utility in the differential diagnosis and relationship with other biomarkers. Alzheimers Res. Ther. 2022, 14, 9. [Google Scholar] [CrossRef]
- Kessel, J.C.; Weiskirchen, R.; Schröder, S.K. Expression analysis of lipocalin 2 (LCN2) in reproductive and non-reproductive tissues of Esr1-deficient mice. Int. J. Mol. Sci. 2023, 24, 9280. [Google Scholar] [CrossRef]
- Xie, S.; Xu, J.; Ma, W.; Liu, Q.; Han, J.; Yao, G.; Huang, X.; Zhang, Y. Lcn5 promoter directs the region-specific expression of cre recombinase in caput epididymidis of transgenic mice. Biol. Reprod. 2013, 88, 71. [Google Scholar] [CrossRef]
- Bratt, T. Lipocalins and cancer. Biochim. Biophys. Acta 2000, 1482, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Živalj, M.; Van Ginderachter, J.A.; Stijlemans, B. Lipocalin-2: A nurturer of tumor progression and a novel candidate for targeted cancer therapy. Cancers 2023, 15, 5159. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Sánchez, G.S.; Pita-Grisanti, V.; Quiñones-Díaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejía, P.E. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int. J. Mol. Sci. 2020, 21, 4365. [Google Scholar] [CrossRef] [PubMed]
- Villodre, E.S.; Hu, X.; Larson, R.; Finetti, P.; Gomez, K.; Balema, W.; Stecklein, S.R.; Santiago-Sanchez, G.; Krishnamurthy, S.; Song, J.; et al. Lipocalin 2 promotes inflammatory breast cancer tumorigenesis and skin invasion. Mol. Oncol. 2021, 15, 2752–2765. [Google Scholar] [CrossRef]
- Flammia, R.S.; Tufano, A.; Proietti, F.; Gerolimetto, C.; DE Nunzio, C.; Franco, G.; Leonardo, C. Renal surgery for kidney cancer: Is preoperative proteinuria a predictor of functional and survival outcomes after surgery? A systematic review of the literature. Minerva Urol. Nephrol. 2022, 74, 255–264. [Google Scholar] [CrossRef]





| Protein | Alias | Chromosomal Localization | Predicted Function |
|---|---|---|---|
| LCN1 | Tear lipocalin (TLC), tear prealbumin (TP), protein migration faster than albumin (PMFA); von Ebner gland protein (VEGP) | 9q34 | Removal of potentially harmful lipids, overproduced in infection ad stress, tear lipocalin |
| LCN2 | Neutrophil gelatinase-associated lipocalin (NGAL), oncogenic lipocalin (24p3), uterocalin, MSFI, siderocalin (Scn) | 9q34.11 | Transport of small lipophilic substances, innate immunity, iron metabolism |
| LCN6 | LCN5, hLcn5, UNQ643/PRO1273 | 9q34.3 | Epididymal lipocalin involved in male fertility and single fertilization |
| LCN8 | EP17, Epididymis secretory sperm binding protein, LCN5, Chromosome 9 open reading frame 137 | 9q34.3 | Sperm maturation via transport of small hydrophobic molecules, male fertility, retinoid carrier protein within epididymis |
| LCN9 | Epididymis luminal protein 129 (HEL129), epididdymal-specific lipocalin-9, 9230102I19Rik, MUP-like lipocalin | 9q34.3 | Binding small hydrophobic ligands |
| LCN10 | 9230112J07Rik, Epididymal-specific lipocalin-10 | 9q34.3 | Protects against inflammation triggered vascular leakage. |
| LCN12 | Epididymal-specific lipocalin-12, MGC48935, epididymis secretory sperm binding protein | 9q34.3 | Male fertility, binds all-trans retinoic acid within epididymis |
| LCN15 | PRO6093, UNQ2541 | 9q34.3 | Olfactory mucus, transport of vitamins, nucleosides |
| A1M | α1-microglobulin, α1-microglobulin/bikunin precursor (AMBP), heterogenous charge protein (HCP), complex-forming glycoprotein heterogeneous in charge, ITI, UTI, EDC1, HI30, ITIL, IATIL, ITILC, uristatin, uronic-acid-rich protein, trypstatin, growth-inhibiting protein 19, MGC64242, AI194774, DKFZp470D2211 | 9q32-q33 | Antioxidant, heme binding, radical scavenging |
| PTGDS | Prostaglandin D2 synthase, PGD2, PDG2 synthase, (PDS, PGDS, PGDS2), lipocalin-type prostaglandin D synthase (LPGDS, L-PGDS) | 9q34.2-q34.3 | Catalyzes PGD2 production and transports lipophilic substances |
| ORM1 | Orosomucoid 1 (ORM), α-1-AGP (AGP1), α- 1-glycoprotein α (AGP-A), HEL-S-153w | 9q32 | Tissue homeostasis and remodeling, acute phase reactant |
| ORM2 | Orosomucoid 2, α-1-acid glycoprotein type 2 (AGP2), α1-glycoprotein β (AGP-B) | 9q32 | Acute phase reactant, immunomodulation and drug delivery, biomarker in cancers |
| OBP2A | Odorant-binding protein 2A, ddorant-binding protein (OBP), LCN13, OBP2C, OBPIIa, HOBPIIa | 9q34 | Scavenger of toxic odors, transport of hydrophobic molecules to olfactory receptors |
| OBP2B | Odorant-binding protein-2B, LCN14, OBPIIb | 9q34 | Chemosensory behavior |
| C8G | Complement component 8, γ subunit | 9q34.3 | Formation of membrane attack complex of the complement |
| PAEP | Progestagen-associated endometrial protein, glycodelin-S (GD-S), glycodelin-A (GdA); glycodelin-F (GdF), glycodelin-S (GdS), PEP; progestagen-dependent endometrial protein (PAEG), placental protein 14 (PP14) | 9q34 | Cell recognition, epithelial differentiation |
| RBP4 | Retinol-binding protein-4, retinol-binding protein, retinal dystrophy iris coloboma and comedogenic acne syndrome protein (RDCCAS), microphthalmia/coloboma 10 (MCOPCB10) | 10q23.33 | Transport of the all-trans form of vitamin A |
| ApoD | Apolipoprotein D | 3q29 | Lipid metabolism, neuroprotection |
| ApoM | Apolipoprotein M, G3a, NG20, HSPC336 | 6p21 | Anti-atherosclerotic, cholesterol efflux |
| Lipocalin | Disease |
|---|---|
| LCN1 | Decreased levels are associated with Sjogren’s syndrome, laser-assisted in situ keratomileusis (LASIK)-induced dry eye, and diabetic retinopathy. Increased expression is seen in cystic fibrosis. |
| LCN2 | Increased expression in insulin resistance, obesity, and inflammatory processes. |
| A1M | Increased levels in proximal tubule defects. Upregulation of A1M protects the skin from damage caused by heme and reactive oxygen species. |
| ApoD | Increased expression associated with altered lipid metabolism, aging, and neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease. |
| C8G | Deficiency of C8G is associated with rare recurrent infections of Neisseria meningitis. It controls bacterial infections by scavenging iron-containing siderophores. |
| PAEP | Decreased levels of PAEP are associated with first trimester abortion, while an increase is seen in gynecological malignancies, melanoma, and lung cancers. |
| PTGDS | Increase in attention deficit hyperactivity disorder and malignancies. |
| RBP4 | Increased in obesity, insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease. |
| LCN13 | Decreased expression in obesity and impact on liver lipid metabolism and fatty acid oxidation and insulin sensitivity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Structure, Functions, and Implications of Selected Lipocalins in Human Disease. Int. J. Mol. Sci. 2024, 25, 4290. https://doi.org/10.3390/ijms25084290
Chandrasekaran P, Weiskirchen S, Weiskirchen R. Structure, Functions, and Implications of Selected Lipocalins in Human Disease. International Journal of Molecular Sciences. 2024; 25(8):4290. https://doi.org/10.3390/ijms25084290
Chicago/Turabian StyleChandrasekaran, Preethi, Sabine Weiskirchen, and Ralf Weiskirchen. 2024. "Structure, Functions, and Implications of Selected Lipocalins in Human Disease" International Journal of Molecular Sciences 25, no. 8: 4290. https://doi.org/10.3390/ijms25084290
APA StyleChandrasekaran, P., Weiskirchen, S., & Weiskirchen, R. (2024). Structure, Functions, and Implications of Selected Lipocalins in Human Disease. International Journal of Molecular Sciences, 25(8), 4290. https://doi.org/10.3390/ijms25084290