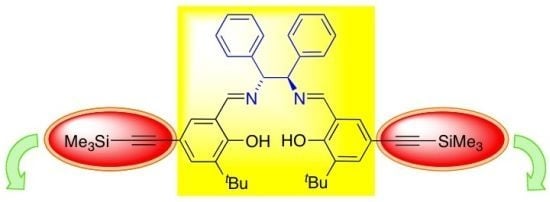
6,6'-(1E,1'E)-((1R,2R)-1,2-Diphenylethane-1,2-diyl)bis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)bis(2-tert-butyl-4-((trimethylsilyl)ethynyl)phenol)
Abstract
:Introduction
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References
- Larrow, J.R.; Jacobsen, E.N. Asymmetric processes catalyzed by chiral (salen)metal complexes. Top. Organomet. Chem. 2004, 6, 123–152. [Google Scholar] [CrossRef]
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by (salen)Manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Irie, R.; Noda, K.; Ito, Y.; Matsumoto, N.; Katsuki, T. Catalytic asymmetric epoxidation of unfunctionalized olefins. Tetrahedron Lett. 1990, 31, 7345–7348. [Google Scholar] [CrossRef]
- McGarrigle, E.M.; Gilheany, D.G. Chromium- and manganese-salen promoted epoxidation of alkenes. Chem. Rev. 2005, 105, 1563–1602. [Google Scholar] [CrossRef] [PubMed]
- Holbach, M.; Weck, M. Modular approach for the development of supported monofunctionalized, salen catalysts. J. Org. Chem. 2006, 71, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Heckel, A.; Seebach, D. Enantioselective heterogeneous epoxidation and hetero-Diels-Alder reaction with Mn- and Cr-salen complexes immobilized on silica gel by radical grafting. Helv. Chim. Acta 2002, 85, 913–926, and references therein. [Google Scholar] [CrossRef]
- Patai, S. Chemistry of Triple-Bonded Functional Groups; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. Acetylene Chemistry: Chemistry, Biology, and Material Science; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Sellner, H.; Karjalainen, J.K.; Seebach, D. Preparation of dendritic and non-dendritic styryl-substituted salens for cross-linking suspension copolymerization with styrene and multiple use of the corresponding Mn and Cr complexes in enantioselective epoxidations and hetero-Diels-Alder reactions. Chem. Eur. J. 2001, 7, 2873–2887. [Google Scholar] [CrossRef]
- Jammi, S.; Rout, L.; Punniyamurthy, T. Chiral linear polymers bonded alternatively with salen and 1,4-dialkoxy-2,6-diethynylbenzene: Synthesis and application to diethylzinc addition to aldehydes. Tetrahedron Asymmetry 2007, 18, 2016–2020. [Google Scholar] [CrossRef]
- Lowe, A.B.; Hoyle, C.E.; Bowman, C.N. Thiol-yne click chemistry: A powerful and versatile methodology for materials synthesis. J. Mater. Chem. 2010, 20, 4745–4750. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huber, T.; Niehues, L.; Cativiela, C.; Finn, M.G.; Díaz, D.D. 6,6'-(1E,1'E)-((1R,2R)-1,2-Diphenylethane-1,2-diyl)bis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)bis(2-tert-butyl-4-((trimethylsilyl)ethynyl)phenol). Molbank 2013, 2013, M795. https://doi.org/10.3390/M795
Huber T, Niehues L, Cativiela C, Finn MG, Díaz DD. 6,6'-(1E,1'E)-((1R,2R)-1,2-Diphenylethane-1,2-diyl)bis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)bis(2-tert-butyl-4-((trimethylsilyl)ethynyl)phenol). Molbank. 2013; 2013(1):M795. https://doi.org/10.3390/M795
Chicago/Turabian StyleHuber, Thimo, Lennart Niehues, Carlos Cativiela, M. G. Finn, and David Díaz Díaz. 2013. "6,6'-(1E,1'E)-((1R,2R)-1,2-Diphenylethane-1,2-diyl)bis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)bis(2-tert-butyl-4-((trimethylsilyl)ethynyl)phenol)" Molbank 2013, no. 1: M795. https://doi.org/10.3390/M795
APA StyleHuber, T., Niehues, L., Cativiela, C., Finn, M. G., & Díaz, D. D. (2013). 6,6'-(1E,1'E)-((1R,2R)-1,2-Diphenylethane-1,2-diyl)bis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)bis(2-tert-butyl-4-((trimethylsilyl)ethynyl)phenol). Molbank, 2013(1), M795. https://doi.org/10.3390/M795