Novel One-Pot Synthesis of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate: Synthetic and Crystallographic Studies
Abstract
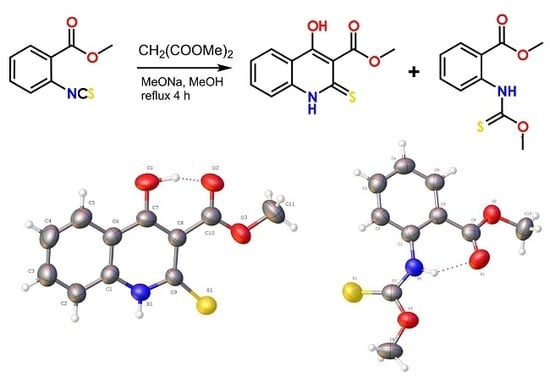
:1. Introduction
2. Results and Discussions
2.1. Synthesis
2.2. X-ray Analysis
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Reaction of Methyl 2-Isothiocyanatobenzoate 12 with Dimethyl Malonate with the Presence of Sodium Methanolate
3.2.2. Synthesis of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate 13
3.2.3. Synthesis of Methyl 2-(Methoxycarbonothioylamino)benzoate 14
3.3. X-ray Diffraction Study
3.3.1. Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate 13
3.3.2. Methyl 2-(Methoxycarbonothioylamino)benzoate 14
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kazuno, K.; Kise, M.; Kitano, M.; Ozaki, M.; Segawa, J.; Shirahase, I.; Tomii, Y. Preparation of Oxothiazetoquinolinecarboxylates as Bactericides. GB Patent 2190376B, 18 November 1987. [Google Scholar]
- Taguchi, M.; Kondo, H.; Inoue, Y.; Kawahata, Y.; Tsukamoto, G. Epoxymethanothiazoloquinolonecarboxylic Acid Derivatives as Medical Bactericides, Their Preparation, and Formulations Containing Them. EPA 286089A1, 12 October 1988. [Google Scholar]
- Kise, M.; Kitano, M.; Ozaki, M.; Kazuno, K.; Matsuda, M.; Shirahase, I.; Segawa, J. Preparation and Testing of 6-Fluoro-7-piperazino-4-oxo-4H-[1,3]thiazeto[3,2-a]quinolinecarboxylate Derivatives as Antibactericides. EPA 315828A1, 17 May 1989. [Google Scholar]
- Segawa, J.; Kitano, M.; Kazuno, K.; Tsuda, M.; Shirahase, I.; Ozaki, M.; Matsuda, M.; Kise, M. Studies on pyridonecarboxylic acids. 2. Synthesis and antibacterial activity of 8-substituted 7-fluoro-5-oxo-5H-thiazolo[3,2-a]quinoline-4-carboxylic acids. J. Heterocycl. Chem. 1992, 29, 1117–1123. [Google Scholar] [CrossRef]
- Segawa, J.; Kazuno, K.; Matsuoka, M.; Shirahase, I.; Ozaki, M.; Matsuda, M.; Tomii, Y.; Kitano, M.; Kise, M. Studies on pyridonecarboxylic acids. III. Synthesis and antibacterial activity evaluation of 1,8-disubstituted 6-fluoro-4-oxo-7-piperazinyl-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid derivatives. Chem. Pharm. Bull. 1995, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Daneshtalab, M. Polycyclic quinolones (part 1) - Thieno[2,3-b]benzo[h]quinoline derivatives: Design, synthesis, preliminary in vitro and in silico studies. Heterocycles 2012, 85, 103–122. [Google Scholar] [CrossRef]
- Segawa, J. Process for Producing Quinolinecarboxylic Acid Derivative. WO 9404506A1, 3 March 1994. [Google Scholar]
- Ivachtchenko, A.; Kovalenko, S.; Drushlyak, O. Synthesis of substituted 4-oxo-2-thioxo-1,2,3,4-tetrahydroquinazolines and 4-oxo-3,4-dihydroquinazoline-2-thioles. J. Comb. Chem. 2003, 5, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Urleb, U. The reactions of heterocyclic isothiocyanates bearing an ortho ester group with N-nucleophiles. The scope and some limitations of the reaction. J. Heterocycl. Chem. 1995, 32, 69–71. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; Bayomi, A.A. Utility of methyl 2-isothiocyanatobenzoate in the synthesis of some new quinazoline derivatives as potential anticancer and radiosensitizing agents. Arzneimittelforschung 2011, 61, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ganesh, T.; Diebold, B.A.; Zhu, Y.; McCoy, J.W.; Smith, S.M.E.; Sun, A.; Lambeth, J.D. Thioxo-dihydroquinazolin-one Compounds as Novel Inhibitors of Myeloperoxidase. ACS Med. Chem. Lett. 2015, 6, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherbuliez, E.; Willhalm, B.; Jaccard, S.; Rabinowitz, J. Formation and rearrangement of esters. LXXVIII. Reaction of o-methoxycarbonylphenylisothiocyanate with diamines and hydrazine, and cyclization of some N-phenyl-N’-hydroxyalkylthioureas. Helv. Chim. Acta 1967, 50, 2563–2569. [Google Scholar] [CrossRef]
- Pathak, U.S.; Alagarsamy, V. Synthesis and pharmacological investigation of some 2-substituted [1,3,4]thiadiazolo[2,3-b]quinazolin-5(4H)-ones as antihypertensive agents. Indian Drugs 2000, 37, 51–55. [Google Scholar]
- Gakhar, H.K.; Shama, A.; Gill, G.K. Thiazolo[2’,3’:3,4]-s-triazolo[5,1-b]quinazolin-10-ones. J. Indian Chem. Soc. 1983, 60, 803–804. [Google Scholar]
- Verma, J.K. Coordination polymers of cobalt(II), nickel(II), copper(II), zinc(II) and cadmium(II) with 3-allyl-1-(2-mercapto-4-oxo-3(4H)-quinazolinyl)-2-thiopseudourea. Curr. Sci. 1987, 56, 1168–1171. [Google Scholar]
- Il’chenko, O.V.; Zaremba, O.V.; Kovalenko, S.M.; Sherakov, A.A.; Chernykh, V.P. Synthesis of 3-Substituted 2-Thioxo-2,3-dihydro-1H-benzofuro[3,2-d]pyrimidin-4(1H)-ones. Synth. Commun. 2007, 37, 2559–2568. [Google Scholar] [CrossRef]
- Tkachenko, O.V.; Vlasov, S.V.; Kovalenko, S.M.; Zhuravel, I.O.; Chernykh, V.P. Synthesis and the antimicrobial activity of ethyl 5-methyl-2-(alkylthio)-4-oxo-3,4-dihydrothieno [2,3-d]pyrimidine-6-carboxylates. Zh. Org. Farm. Khim. 2013, 11, 9–15. [Google Scholar] [CrossRef]
- Parkhomenko, O.O.; Kovalenko, S.M.; Chernykh, V.P.; Osolodchenko, T.P. Synthesis and antimicrobial activity of 5-oxo-1-thioxo-4,5-dihydro[1,3]thiazolo[3,4-a]quinazolines. ARKIVOC 2005, 8, 82–88. [Google Scholar] [CrossRef]
- Burgi, H.-B.; Dunitz, J.D. Structure Correlation; VCH: Weinheim, Germany, 1994; Volume 2, p. 741. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalenko, S.M.; Drushlyak, O.G.; Konovalova, I.S.; Mariutsa, I.O.; Kravchenko, D.V.; Ivachtchenko, A.V.; Mitkin, O.D. Novel One-Pot Synthesis of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate: Synthetic and Crystallographic Studies. Molbank 2019, 2019, M1085. https://doi.org/10.3390/M1085
Kovalenko SM, Drushlyak OG, Konovalova IS, Mariutsa IO, Kravchenko DV, Ivachtchenko AV, Mitkin OD. Novel One-Pot Synthesis of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate: Synthetic and Crystallographic Studies. Molbank. 2019; 2019(4):M1085. https://doi.org/10.3390/M1085
Chicago/Turabian StyleKovalenko, Sergiy M., Oleksandr G. Drushlyak, Irina S. Konovalova, Illia O. Mariutsa, Dmitry V. Kravchenko, Alexandre V. Ivachtchenko, and Oleg D. Mitkin. 2019. "Novel One-Pot Synthesis of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate: Synthetic and Crystallographic Studies" Molbank 2019, no. 4: M1085. https://doi.org/10.3390/M1085
APA StyleKovalenko, S. M., Drushlyak, O. G., Konovalova, I. S., Mariutsa, I. O., Kravchenko, D. V., Ivachtchenko, A. V., & Mitkin, O. D. (2019). Novel One-Pot Synthesis of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate: Synthetic and Crystallographic Studies. Molbank, 2019(4), M1085. https://doi.org/10.3390/M1085