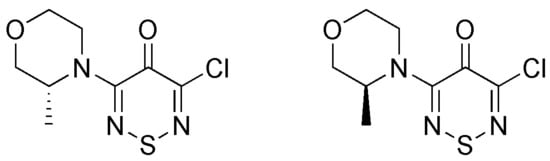
Synthesis of (R) and (S)-3-Chloro-5-(3-methylmorpholino)-4H-1,2,6-thiadiazin-4-ones
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2020, 40, 709–752. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Yin, B.; Wu, Y. Garland chrysanthemum analogue, its synthesis method and use. Chinese Patent 1,431,210, 24 January 2003. [Google Scholar]
- Saravana, L.A.K.; Mayakrishnan, G.; Moorthy, S.K.; Anandaram, S. 2-Acetylpyridine-N(4)-morpholine thiosemicarbazone (HAcpMTSc) as a corrosion inhibitor on mild steel in HCl. Ind. Eng. Chem. Res. 2011, 50, 7824–7832. [Google Scholar] [CrossRef]
- Muhsin, M.; Graham, J.; Kirkpatrick, P. Gefitinib. Nat. Rev. Drug Discovery 2003, 2, 515–516. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Q.; Fang, L. Design and synthesis of alkyl substituted pyridino [2,3-d]pyrimidine compounds as PI3Kα/mTOR dual inhibitors with improved pharmacokinetic properties and potent in vivo antitumor activity. Bioorg. Med. Chem. 2018, 26, 3992–4000. [Google Scholar] [CrossRef]
- Vargas, B.; Giacobbi, N.S.; Venkatachari, N.J.; Han, F.; Sluis-Cremer, N.; Sanyal, A.; Gupta, P. Inhibitors of signaling pathways that block reversal of HIV-1 latency. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, D.J.; St Jean, D.J., Jr.; Kurzeja, R.J.; Wahl, R.C.; Michelsen, K.; Cupples, R.; Chen, M.; Wu, J.; Sivits, G.; Helmering, J.; et al. Antidiabetic effects of glucokinase regulatory protein small-molecule disruptors. Nature 2013, 504, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Rageot, D.; Bohnacker, T.; Keles, E.; McPhail, J.A.; Hoffmann, R.M.; Melone, A.; Borsari, C.; Sriramaratnam, R.; Sele, A.M.; Beaufils, F.; et al. (S)-4-(Difluoromethyl)-5-(4-(3-methylmorpholino)-6-morpholino-1,3,5-triazin-2-yl)pyridin-2-amine (PQR530), a potent, orally bioavailable, and brain-penetrable dual inhibitor of class I PI3K and mTOR kinase. J. Med. Chem. 2019, 62, 6241–6261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Z.; Blackwood, E.; Liu, L.; Malek, S.; Belvin, M.; Koehler, M.F.; Ortwine, D.F.; Chen, H.; Cohen, F.; Kenny, J.R.; et al. Discovery and biological profiling of potent and selective mTOR inhibitor GDC-0349. ACS Med. Chem. Lett. 2013, 29, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Guichard, S.M.; Curwen, J.; Bihani, T.; D’Cruz, C.M.; Yates, J.W.; Grondine, M.; Howard, Z.; Davies, B.R.; Bigley, G.; Klinowska, T.; et al. AZD2014, an inhibitor of mTORC1 and mTORC2, is highly effective in ER+ breast cancer when administered using intermittent or continuous schedules. Mol. Cancer Ther. 2015, 14, 2508–2518. [Google Scholar] [CrossRef] [Green Version]
- Wienen-Schmidt, B.; Schmidt, D.; Gerber, H.D.; Heine, A.; Gohlke, H.; Klebe, G. Surprising non-additivity of methyl groups in drug–kinase interaction. ACS Chem. Biol. 2019, 14, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Pisa, R.; Cupido, T.; Kapoor, T.M. Designing allele-specific inhibitors of spastin, a microtubule-severing AAA protein. J. Am. Chem. Soc. 2019, 141, 5602–5606. [Google Scholar] [CrossRef]
- Pettersson, M.; Hou, X.; Kuhn, M.; Wager, T.T.; Kauffman, G.W.; Verhoest, P.R. Quantitative assessment of the impact of fluorine substitution on P-Glycoprotein (P-gp) mediated efflux, permeability, lipophilicity, and metabolic stability. J. Med. Chem. 2016, 59, 5284–5296. [Google Scholar] [CrossRef] [PubMed]
- Degorce, S.L.; Bodnarchuk, M.S.; Scott, J.S. Lowering lipophilicity by adding carbon: Azaspiroheptanes, a logD lowering twist. ACS Med. Chem. Lett. 2019, 10, 1198–1204. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Laitinen, T.; Bennett, J.M.; Wells, C.I.; Elkins, J.M.; Zuercher, W.J.; Tizzard, G.J.; Poso, A. Design and analysis of the 4-anilinoquin(az)oline kinase inhibition profiles of GAK/SLK/STK10 using quantitative structure-activity relationships. ChemMedChem 2020, 15, 26–49. [Google Scholar] [CrossRef] [Green Version]
- Toenjes, S.T.; Garcia, V.; Maddox, S.M.; Dawson, G.A.; Ortiz, M.A.; Piedrafita, F.J.; Gustafson, J.A. Leveraging atropisomerism to obtain a selective inhibitor of RET kinase with secondary activities toward EGFR mutants. ACS Chem. Biol. 2019, 14, 1930–1939. [Google Scholar] [CrossRef]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,097,594A, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-thio-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,100,281A, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. 3-Chloro-5-(optionally substituted heterocycloxy)-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,143,138, 3 March 1979. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,201,780, 6 May 1980. [Google Scholar]
- Portnoy, R.C. Thiadiazinone plant disease control agents. U.S. Patent 4,497,807A, 5 February 1985. [Google Scholar]
- Gómez, T.; Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, cyclohepta- and cyclopentadithiazoles: New materials and a rich heterocyclic chemistry of cyclic enaminonitriles. Eur. J. Org. Chem. 2005, 5055–5066. [Google Scholar] [CrossRef]
- Chochos, C.L.; Kalogirou, A.S.; Ye, T.; Tatsi, E.; Katsouras, A.; Zissimou, G.A.; Gregoriou, V.G.; Avgeropoulos, A.; Koutentis, P.A. 4H-1,2,6-Thiadiazine-containing donor–acceptor conjugated polymers: Synthesis, optoelectronic characterization and their use in organic solar cells. J. Mater. Chem. C 2018, 6, 3658–3667. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Godoi, P.H.; Couñago, R.M.; Laitinen, T.; Scott, J.W.; Langendorf, C.G.; Oakhill, J.S.; Drewry, D.H.; Zuercher, W.J.; Koutentis, P.A.; et al. 1,2,6-Thiadiazinones as Novel Narrow Spectrum Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) Inhibitors. Molecules 2018, 23, 1221. [Google Scholar] [CrossRef] [Green Version]
- Kalogirou, A.S.; Koutentis, P.A. The chemistry of non-S-oxidised 4H-1,2,6-thiadiazines. Targets Heterocycl. Syst. 2018, 22, 82–118. [Google Scholar] [CrossRef]
- Geevers, J.; Trompen, W.P. Synthesis and reactions of 3,5-dichloro-4H-1,2,6-thiadiazin-4-one. Recl. Trav. Chim. Pays.-Bas. 1974, 93, 270–272. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Koutentis, P.A. Synthesis of asymmetric 3,5-diaryl-4H-1,2,6-thiadiazin-4-ones via Suzuki–Miyaura and Stille coupling reactions. Tetrahedron 2012, 68, 7380–7385. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Selective Stille coupling reactions of 3-chloro-5-halo(pseudohalo)-4H-1,2,6-thiadiazin-4-ones. Org. Lett. 2011, 13, 5886–5889. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Palladium catalyzed C-C coupling reactions of 3,5-dichloro-4H-1,2,6-thiadiazin-4-one. Org. Lett. 2011, 13, 3466–3469. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. Pd-catalyzed C-N coupling of primary (het)arylamines with 5-substituted 3-chloro-4H-1,2,6-thiadiazin-4-ones. Tetrahedron Lett. 2018, 59, 2653–2656. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. A qualitative comparison of the reactivities of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine and 4,5-dichloro-1,2,3-dithiazolium chloride. Molecules 2015, 20, 14576–14594. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Asquith, C.R.M.; Koutentis, P.A. Synthesis of (R) and (S)-3-Chloro-5-(3-methylmorpholino)-4H-1,2,6-thiadiazin-4-ones. Molbank 2020, 2020, M1128. https://doi.org/10.3390/M1128
Kalogirou AS, Asquith CRM, Koutentis PA. Synthesis of (R) and (S)-3-Chloro-5-(3-methylmorpholino)-4H-1,2,6-thiadiazin-4-ones. Molbank. 2020; 2020(2):M1128. https://doi.org/10.3390/M1128
Chicago/Turabian StyleKalogirou, Andreas S., Christopher R. M. Asquith, and Panayiotis A. Koutentis. 2020. "Synthesis of (R) and (S)-3-Chloro-5-(3-methylmorpholino)-4H-1,2,6-thiadiazin-4-ones" Molbank 2020, no. 2: M1128. https://doi.org/10.3390/M1128
APA StyleKalogirou, A. S., Asquith, C. R. M., & Koutentis, P. A. (2020). Synthesis of (R) and (S)-3-Chloro-5-(3-methylmorpholino)-4H-1,2,6-thiadiazin-4-ones. Molbank, 2020(2), M1128. https://doi.org/10.3390/M1128