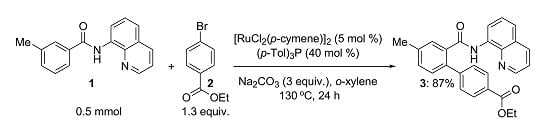
4’-Methyl-2’-(quinolin-8-ylcarbamoyl)-biphenyl-4-carboxylic Acid Ethyl Ester
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
General Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shilov, A.E.; Shul’pin, G.B. Activation of C-H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Q.; Shi, Z. CH Activation, Topic in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; p. 292. [Google Scholar]
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild metal-catalyzed C–H activation: Examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeldt, S.R.; Sanford, M.S. Controlling Site Selectivity in Palladium-Catalyzed C–H Bond Functionalization. Acc. Chem. Res. 2012, 45, 936–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omae, I. Intramolecular five-membered ring compounds and their applications. Coord. Chem. Rev. 2004, 248, 995–1023. [Google Scholar] [CrossRef]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnio, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A Comprehensive review of directing groups applied in metal-catalyzed C-H functionalization chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [Green Version]
- Rouquet, G.; Chatani, N. Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef]
- Rej, S.; Ano, Y.; Chatani, N. Bidentate Directing Groups: An Efficient Tool in C–H Bond Functionalization Chemistry for the Expedient Construction of C–C Bonds. Chem. Rev. 2020, 120, 1788–1887. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.Q. Palladium catalyzed transformations of alkyl C−H bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef]
- Davies, H.M.L.; Walji, A.M. Modern Rhodium-Catalyzed Organic Reactions; Evans, P.A., Ed.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-catalyzed C-C bond formation via heteroatom-directed C-H bond activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [Green Version]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-Catalyzed C–H Bond Activation and Functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef] [PubMed]
- Khake, S.M.; Chatani, N. Chelation-assisted Ni-catalyzed C-H functionalizations. Trends Chem. 2019, 1, 524–539. [Google Scholar] [CrossRef]
- Chatani, N. Nickel-Catalyzed C–H bond functionalization utilizing an N,N-bidentate directing group. Top. Organomet. Chem. 2016, 56, 9–46. [Google Scholar]
- Shang, R.; Ilies, L.; Nakamura, E. Iron-catalyzed C-H bond activation. Chem. Rev. 2017, 117, 9086–9139. [Google Scholar] [CrossRef] [PubMed]
- Weiping, L.; Ackermann, L. Manganese-catalyzed C-H activation. ACS Catal. 2016, 6, 3743–3752. [Google Scholar]
- Sato, T.; Yoshida, T.; Al Mamari, H.H.; Ilies, L.; Nakamura, E. Manganese-catalyzed directed methylation of C(sp2)-H bonds at 25 °C with high catalytic turnover. Org. Lett. 2017, 19, 5458–5461. [Google Scholar] [CrossRef]
- Aihara, Y.; Chatani, N. Ruthenium-catalyzed direct arylation of C–H bonds in aromatic amides containing a bidentate directing group: Significant electronic effects on arylation. Chem. Sci. 2013, 4, 664–670. [Google Scholar] [CrossRef]
- Al Mamari, H.H.; Diers, E.; Ackermann, L. Triazole-assisted ruthenium-catalyzed C-H arylation of aromatic amides. Chem. Eur. J. 2014, 20, 9739–9743. [Google Scholar] [CrossRef]
- Gu, Q.; Al Mamari, H.H.; Graczyk, K.; Diers, E.; Ackermann, L. Iron-catalyzed C(sp2)-H and C(sp3)-H arylation by triazole assistance. Angew. Chem. Int. Ed. 2014, 53, 3868–3871, Angew. Chem. 2014, 126, 3949–3952. [Google Scholar] [CrossRef]
- Al Mamari, H.H.; Al Awaimri, N.; Al Lawati, Y. N-Benzo[c][1,2,5]thiazol-4-yl-3-trifluoromethylbenzamide. Molbank 2019, 2019, M1075. [Google Scholar] [CrossRef] [Green Version]
- Al Mamari, H.H.; Al Lawati, Y. N-(2-Hydroxy-1,1-dimethylethyl)-3-methylbenzamide. Molbank 2020, 2020, M1099. [Google Scholar] [CrossRef] [Green Version]
- Shibata, K.; Yamaguchi, T.; Chatani, N. Rhodium-Catalyzed Alkylation of C–H Bonds in Aromatic Amides with Styrenes via Bidentate–Chelation Assistance. Org. Lett. 2015, 17, 3584–3587. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamari, H.H.A.; Hasani, A.A. 4’-Methyl-2’-(quinolin-8-ylcarbamoyl)-biphenyl-4-carboxylic Acid Ethyl Ester. Molbank 2020, 2020, M1132. https://doi.org/10.3390/M1132
Mamari HHA, Hasani AA. 4’-Methyl-2’-(quinolin-8-ylcarbamoyl)-biphenyl-4-carboxylic Acid Ethyl Ester. Molbank. 2020; 2020(2):M1132. https://doi.org/10.3390/M1132
Chicago/Turabian StyleMamari, Hamad H. Al, and Anfal Al Hasani. 2020. "4’-Methyl-2’-(quinolin-8-ylcarbamoyl)-biphenyl-4-carboxylic Acid Ethyl Ester" Molbank 2020, no. 2: M1132. https://doi.org/10.3390/M1132
APA StyleMamari, H. H. A., & Hasani, A. A. (2020). 4’-Methyl-2’-(quinolin-8-ylcarbamoyl)-biphenyl-4-carboxylic Acid Ethyl Ester. Molbank, 2020(2), M1132. https://doi.org/10.3390/M1132