Unexpected Formation of 4-[(1-Carbamoyl-3-oxo-1,3-dihydro-2-benzofuran-1-yl)amino]benzoic Acid from 4-[(3-Amino-1-oxo-1H-2-benzopyran-4-yl)amino]benzoic Acid
Abstract
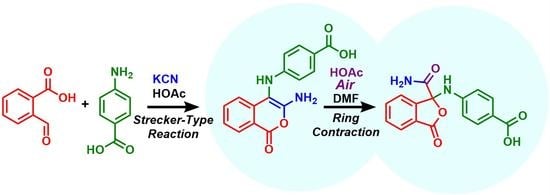
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Preparation of 4-[(3-Amino-1-oxo-1H-2-benzopyran-4-yl)amino]benzoic Acid (10a)
4.3. Preparation of 4-[(1-Carbamoyl-3-oxo-1,3-dihydro-2-benzofuran-1-yl)amino]benzoic Acid (11a)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kihampa, C.; Nkunya, M.H.H.; Joseph, C.C.; Magesa, S.M.; Hassanali, A.; Heydenreich, M.; Kleinpeter, E. Anti-Mosquito and Antimicrobial nor-Halimanoids, Isocoumarins and an Anilinoid from Tessmannia densiflora. Phytochemistry 2009, 70, 1233–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piacente, S.; Pizza, C.; de Tommasi, N.; Vittorio Emanuele, P.; Fisciano, D.; Mahmood, N. Constituents of Ardisia japonica and Their In Vitro Anti-HIV Activity. J. Nat. Prod. 1996, 59, 565–569. [Google Scholar] [CrossRef]
- Zeng, W.N.; Cai, J.; Wang, B.; Chen, L.Y.; Pan, C.X.; Chen, S.J.; Huang, G.L.; Zheng, C.J. A New Bioactive Isocoumarin from the Mangrove-Derived Fungus Penicillium sp. TGM112. J. Asian Nat. Prod. Res. 2021, 24, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Park, J.; Kang, K.B.; Kim, T.B.; Oh, W.K.; Kim, J.; Sung, S.H. Acylphloroglucinolated Catechin and Phenylethyl Isocoumarin Derivatives from Agrimonia pilosa. J. Nat. Prod. 2016, 79, 2376–2383. [Google Scholar] [CrossRef]
- Wijeratne, E.M.K.; Paranagama, P.A.; Gunatilaka, A.A.L. Five New Isocoumarins from Sonoran Desert Plant-associated Fungal Strains Paraphaeosphaeria quadriseptata and Chaetomium chiversii. Tetrahedron 2006, 62, 8439–8446. [Google Scholar] [CrossRef]
- Zhang, H.; Matsuda, H.; Kumahara, A.; Ito, Y.; Nakamura, S.; Yoshikawa, M. New Type of Anti-diabetic Compounds from the Processed Leaves of Hydrangea macrophylla var. thunbergii (Hydrangeae Dulcis Folium). Bioorg. Med. Chem. Lett. 2007, 17, 4972–4976. [Google Scholar] [CrossRef]
- Prompanya, C.; Dethoup, T.; Bessa, L.J.; Pinto, M.M.M.; Gales, L.; Costa, P.M.; Silva, A.M.S.; Kijjoa, A. New Isocoumarin Derivatives and Meroterpenoids from the Marine Sponge-Associated Fungus Aspergillus similanensis sp. Nov. KUFA 0013. Mar. Drugs 2014, 12, 5160–5173. [Google Scholar] [CrossRef]
- Saeed, A.; Haroon, M.; Muhammad, F.; Larik, F.A.; Hesham, E.-S.; Channar, P.A. Advances in Transition Metal Catalyzed Synthesis of 3-substituted Isocoumarins. J. Organomet. Chem. 2017, 834, 88–103. [Google Scholar] [CrossRef]
- Hara, Y.; Onodera, S.; Kochi, T.; Kakiuchi, F. Catalytic Formation of α-Aryl Ketones by C-H Functionalization with Cyclic Alkenyl Carbonates and One-Pot Synthesis of Isocoumarins. Org. Lett. 2015, 17, 4850–4853. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, L.; Tong, Q.; Wu, W.; Jiang, H. Oxypalladation Initiating the Oxidative Heck Reaction with Alkenyl Alcohols: Synthesis of Isocoumarin–Alkanones. Eur. J. Org. Chem. 2016, 2016, 663–667. [Google Scholar] [CrossRef]
- Teixeira, M.; Alvarenga, E.; Lopes, D.; Oliveira, D. Herbicidal activity of isobenzofuranones and in silico identification of their enzyme target. Pest. Manag. Sci. 2019, 75, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Arnone, A.; Assante, G.; Nacini, G.; Strada, S.; Vercesi, A. Cryphonectric Acid and Other Minor Metabolites from a Hypovirulent Strain of Cryphonectria parasitica. J. Nat. Prod. 2002, 65, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, Z.; Yasin, H.; Şenel, P.; Adımcılar, V.; Erdoğan, T.; Özdemir, A.; Gölcü, A.; Odabaşoğlu, M. A novel 3-((5-methylpyridin-2-yl)amino)isobenzofuran-1(3H)-one: Molecular structure describe X-ray diffractions and DFT calculations, antioxidant activity, DNA binding and molecular docking studies. J. Mol. Struct. 2020, 1205, 127585. [Google Scholar] [CrossRef]
- Zou, S.; Wang, Z.; Wang, J.; Wei, G.; Wang, W.; Zang, Y.; Zeng, F.; Chen, K.; Liu, J.; Wang, J.; et al. Azacoccones A-E, five new aza-epicoccone derivatives from Aspergillus flavipes. Fitoterapia 2018, 124, 127–131. [Google Scholar] [CrossRef]
- Hou, Y.-T.; Wu, F.; Yao, J.-H.; Zhu, Z.-H.; Mi, Q.-L.; Gao, Q.; Zhou, M.; Ye, Y.-Q.; Wang, W.-G.; Yang, G.-Y.; et al. Chemical constituents of the roots of Phlomis betonicoides and their anti-rotavirus activity. Chem. Nat. Compd. 2021, 57, 864–868. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Li, K.; Wu, Q.; Chen, R.; Liu, X.; Ruan, B. Novel phthalide derivatives: Synthesis and anti-inflammatory activity in vitro and in vivo. Eur. J. Med. Chem. 2020, 206, 112722. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, J.-J.; He, J.; Feng, T.; Liu, J.-K. Isobenzofuranones and isocoumarins from kiwi endophytic fungus Paraphaeosphaeria sporulosa and their antibacterial activity against Pseudomonas syringae pv. actinidiae. Phytochemistry 2022, 195, 113050. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, Z.; Luo, B.; Lan, Q.; Fan, J.; Xue, W.; Miao, J.; Li, Y.; Tang, L.; Fan, L. Design, Synthesis and Antifungal Activities of 6-Substituted 3-Butylphthalide Derivatives against Phytopathogenic Fungi. Chem. Biodivers. 2020, 17, e200435. [Google Scholar] [CrossRef]
- Sánchez-Fernández, R.; Sánchez-Fuentes, R.; Rangel-Sánchez, H.; Hernández-Ortega, S.; López-Cortés, J.; Macías-Rubalcava, M. Antifungal and antioomycete activities and modes of action of isobenzofuranones isolated from the endophytic fungus Hypoxylon anthochroum strain Gseg1. Pestic. Biochem. Phys. 2020, 169, 104670. [Google Scholar] [CrossRef]
- Rodrigues, M.P.; Tomaz, D.C.; de Souza, L.Â.; Onofre, T.S.; de Menezes, W.A.; Almeida, J.; Suarez, A.M.; de Almeida, M.R.; da Silva, A.M.; Costa, G.; et al. Synthesis of cinnamic acid derivatives and leishmanicidal activity against Leishmania braziliensis. Eur. J. Med. Chem. 2019, 183, 111688. [Google Scholar] [CrossRef]
- Karmakar, R.; Pahari, P.; Mal, D. Phthalides and Phthalans: Synthetic Methodologies and Their Applications in the Total Synthesis. Chem. Rev. 2014, 114, 6213–6284. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, S.; Rajopadhyay, V.; Avaragolla, S.P.; Koul, S.; Ramaiah, P.A.; Bhuniya, D. Synthesis of L-azaisotryptophan and its analogs: A general access to new 2-substituted azaindoles. Tetrahedron Lett. 2014, 55, 2415–2419. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Morrel, A.; Conda-Sheridan, M.; Marchand, C.; Agama, K.; Bermingam, A.; Stephen, A.; Chergui, A.; Naumova, A.; Fisher, R.; et al. Synthesis and Biological Evaluation of the First Dual Tyrosyl-DNA Phosphodiesterase I (Tdp1)–Topoisomerase I (Top1) Inhibitors. J. Med. Chem. 2012, 55, 4457–4478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, O.H.; Tawfeeq, H.M.; Muslim, R.F. Synthesis and Characterization of Novel 1,3-oxazepin-5(1H)-one Derivatives via Reaction of Imine Compounds with Isobenzofuran-1(3H)-one. Acta Pharm. Sci. 2017, 55, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Opatz, T.; Ferenc, D. Facile Preparation of 3-Amino-4-(arylamino)-1H-isochromen-1-ones by a New Multicomponent Reaction. Eur. J. Org. Chem. 2005, 2005, 817–821. [Google Scholar] [CrossRef]
- Opatz, T.; Ferenc, D. Ring Contracting Rearrangements of 3-Amino-4-(arylamino)-1H-isochromen-1-ones. Eur. J. Org. Chem. 2006, 2006, 121–126. [Google Scholar] [CrossRef]






| Entry | Conditions | Time | Yield (%) |
|---|---|---|---|
| 1 | r.t. | 1 week | 52 |
| 2 | HOAc/r.t. | 48 h | 57 |
| 3 | HOAc/60 °C | 48 h | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, R.; Quiroga-Suavita, F.; Dotor Robayo, M.Y. Unexpected Formation of 4-[(1-Carbamoyl-3-oxo-1,3-dihydro-2-benzofuran-1-yl)amino]benzoic Acid from 4-[(3-Amino-1-oxo-1H-2-benzopyran-4-yl)amino]benzoic Acid. Molbank 2022, 2022, M1407. https://doi.org/10.3390/M1407
Rodríguez R, Quiroga-Suavita F, Dotor Robayo MY. Unexpected Formation of 4-[(1-Carbamoyl-3-oxo-1,3-dihydro-2-benzofuran-1-yl)amino]benzoic Acid from 4-[(3-Amino-1-oxo-1H-2-benzopyran-4-yl)amino]benzoic Acid. Molbank. 2022; 2022(3):M1407. https://doi.org/10.3390/M1407
Chicago/Turabian StyleRodríguez, Ricaurte, Felipe Quiroga-Suavita, and Mónica Yadira Dotor Robayo. 2022. "Unexpected Formation of 4-[(1-Carbamoyl-3-oxo-1,3-dihydro-2-benzofuran-1-yl)amino]benzoic Acid from 4-[(3-Amino-1-oxo-1H-2-benzopyran-4-yl)amino]benzoic Acid" Molbank 2022, no. 3: M1407. https://doi.org/10.3390/M1407
APA StyleRodríguez, R., Quiroga-Suavita, F., & Dotor Robayo, M. Y. (2022). Unexpected Formation of 4-[(1-Carbamoyl-3-oxo-1,3-dihydro-2-benzofuran-1-yl)amino]benzoic Acid from 4-[(3-Amino-1-oxo-1H-2-benzopyran-4-yl)amino]benzoic Acid. Molbank, 2022(3), M1407. https://doi.org/10.3390/M1407