Microplastics in Freshwater: What Is the News from the World?
Abstract
:1. Introduction
1.1. Microplastics in Aquatic Ecosystems
1.2. Aim
2. Materials and Methods
2.1. Bibliographic Collection
2.2. Collection of Qualitative Information Data
2.3. fMPs Concentration in Field
3. Results
3.1. Bibliographic Search by Year and Type of Ecosystem
3.2. Study Areas Overview
3.3. Overview of Authors’ Affiliate Countries
3.4. fMPs Topics of Investigation
3.5. fMPs Contamination of Water and Sediment
3.6. fMP Impacts on Wild Biota
3.7. Types of fMPs
4. Discussion
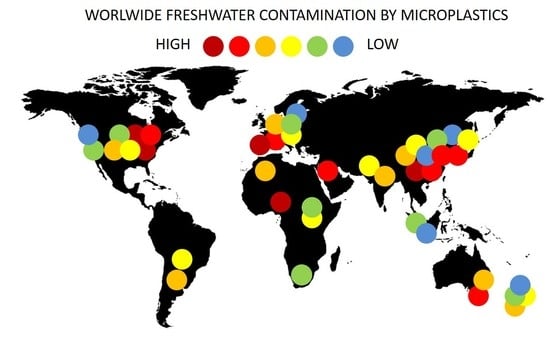
4.1. Geographical Distribution of Contaminated Sites
4.2. Factors Influencing fMP Contamination
4.3. Microplastics in Water and Sediment
4.4. Microplastics in Biota
4.5. Methodological Considerations towards a Standardised Protocol
4.6. The Issue of Identifying Primary and Secondary Microplastics
4.7. Polymer Identification Techniques and Contamination of Fresh and Marine Waters
5. Conclusions
- drafting a standardised freshwater protocol for sampling, analysis, and expression of results, which could facilitate and enhance research on monitoring and risk assessment of fMPs;
- determining global sources of contamination and transport routes based on spatial data, increasing monitoring activities for remote, low-impact or non-sampled areas and temporal changes by improving diachronic studies and, furthermore, specifying if the detected fMPs are primary or secondary would be a valid addition to the choices made by political planners for environmental protection management actions; and
- studying the biota contamination in more detail and the interactions with fMPs (and fMPs-mediated pollutants), in particular regarding benthic organisms, which are generally exposed to higher concentrations (as they are strongly connected to sediments) and trophic web transfer, to clarify the hazard to human health.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, S.; Mukai, H.; Tanabe, S.; Sakayama, K.; Miyazaki, T.; Masuno, H. Butyltin residues in livers of humans and wild terrestrial mammals and in plastic products. Environ. Pollut. 1999, 106, 213–218. [Google Scholar] [CrossRef]
- Lacerda, A.L.D.F.; Rodrigues, L.D.S.; van Sebille, E.; Rodrigues, F.L.; Ribeiro, L.; Secchi, E.R.; Kessler, F.; Proietti, M.C. Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 2019, 9, 3977. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A catchment-scale perspective of plastic pollution. Glob. Chang. Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau. China Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 16–22. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Cheung, P.K.; Hung, P.L.; Fok, L. River Microplastic Contamination and Dynamics upon a Rainfall Event in Hong Kong, China. Environ. Process. 2019, 6, 253–264. [Google Scholar] [CrossRef]
- Piñon-Colin, T.D.J.; Rodriguez-Jimenez, R.; Rogel-Hernandez, E.; Alvarez-Andrade, A.; Wakida, F.T. Microplastics in stormwater runoff in a semiarid region, Tijuana, Mexico. Sci. Total Environ. 2020, 704, 135411. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Fleet, D.; van Franeker, J.; Katsavenakis, S.; Maes, T.; Mouat, J.; Oosterbaan, L.; Poitou, I.; Hanke, G.; Thompson, R.; et al. Marine Strategy Framework Directive Task Team 10 Report Marine Litter; European Union, IFREMER and ICES; Office for Official Publications of the European Communities: Luxemburg, 2010; 48p. [Google Scholar]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the ‘‘plastisphere’’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings - entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Abrial, E.; Khan, F.; Sivri, N.; Espinola, L.A. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res. 2018, 143, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, C.; Puckridge, M.; Schuyler, Q.A.; Townsend, K.; Hardesty, B.D. A quantitative analysis linking sea turtle mortality and plastic debris ingestion. Sci. Rep. 2018, 8, 12536. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.-J.; Kwon, J.-H. Estimating microplastic-bound intake of hydrophobic organic chemicals by fish using measured desorption rates to artificial gut fluid. Sci. Total Environ. 2019, 651, 162–170. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; Mcllwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Franzellitti, S.; Canesi, L.; Auguste, M.; Wathsala, R.H.G.R.; Fabbri, E. Microplastic exposure and effects in aquatic organisms: A physiological perspective. Environ. Toxicol. Pharmacol. 2019, 68, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of plastic debris by rivers into the sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Phoenix, V.; Gauchotte-Lindsay, C. Micro- and nanoplastic pollution of freshwater and wastewater treatment systems. Springer Sci. Rev. 2017, 5, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akindele, E.O.; Ehlers, S.M.; Koop, J.H.E. First empirical study of freshwater microplastics in West Africa using gastropods from Nigeria as bioindicators. Limnologica 2019, 78, 125708. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Kettner, M.T.; Miki, T.; Grossart, H.-P. Microplastics: New substrates for heterotrophic activity contribute to altering organic matter cycles in aquatic ecosystems. Sci. Total Environ. 2018, 635, 1152–1159. [Google Scholar] [CrossRef]
- Hoellein, T.J.; McCormick, A.R.; Hittie, J.; London, M.G.; Scott, J.W.; Kelly, J.J. Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw. Sci. 2017, 36, 491–507. [Google Scholar] [CrossRef]
- Kettner, M.T.; Rojas-Jimenez, K.; Oberbeckmann, S.; Labrenz, M.; Grossart, H.-P. Microplastics alter composition of fungal communities in aquatic ecosystems. Environ. Microbiol. 2017, 9, 4447–4459. [Google Scholar] [CrossRef]
- Kettner, M.T.; Oberbeckmann, S.; Labrenz, M.; Grossart, H.-P. The eukaryotic life on microplastics in brackish ecosystems. Front. Microbiol. 2019, 10, 538. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Collard, F.; Gasperi, J.; Gilbert, B.; Eppe, G.; Azimi, S.; Rocher, V.; Tassin, B. Anthropogenic particles in the stomach contents and liver of the freshwater fish Squalius cephalus. Sci. Total Environ. 2018, 643, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-J.; Lee, S.-H.; Lee, M.-S.; Lee, J.-K.; Lee, S.-H.; Zoh, K.-D. Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci. Total Environ. 2020, 708, 134535. [Google Scholar] [CrossRef]
- Brookson, C.B.; de Solla, S.R.; Fernie, K.J.; Cepeda, M.; Rochmana, C.M. Microplastics in the diet of nestling double-crested cormorants (Phalacrocorax auritus), an obligate piscivore in a freshwater ecosystem. Can. J. Fish. Aquat. Sci. 2019, 76, 2156–2163. [Google Scholar] [CrossRef] [Green Version]
- Faure, F.; Corbaz, M.; Baecher, H.; de Alencastro, L.F. Pollution due to plastics and microplastics in Lake Geneva and in the Mediterranean Sea. Arch. Sci. 2012, 65, 157–164. [Google Scholar]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; de Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Holland, E.; Mallory, M.; Shutler, D. Plastics and other anthropogenic debris in freshwater birds from Canada. Sci. Total Environ. 2016, 571, 251–258. [Google Scholar] [CrossRef]
- Schessl, M.; Johns, C.; Ashpole, S.L. Microbeads in sediment, dreissenid mussels, and anurans in the littoral zone of the upper St. Lawrence River, New York. Pollution 2019, 5, 41–52. [Google Scholar] [CrossRef]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Tot. Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef]
- Simmerman, C.B.; Coleman Wasik, J.K. The effect of urban point source contamination on microplastic levels in water and organisms in a cold-water stream. Limnol. Oceanogr. 2019, 5, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Tibbetts, J.; Krause, S.; Lynch, I.; Smith, G.H.S. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 2018, 10, 1597. [Google Scholar] [CrossRef] [Green Version]
- Eerkes-Medrano, D.; Thompson, R. Occurrence, fate, and effect of microplastics in freshwater systems. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–132. [Google Scholar]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Xiong, X. Microplastic Pollution in Inland Waters Focusing on Asia. In Freshwater Microplastics; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; p. 58. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, M.J.; Hittinger, E. Inventory and transport of plastic debris in the Laurentian Great Lakes. Mar. Pollut. Bull. 2017, 115, 273–281. [Google Scholar] [CrossRef]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef]
- Yin, L.; Wen, X.; Du, C.; Jiang, J.; Wu, L.; Zhang, Y.; Hu, Z.; Hu, S.; Feng, Z.; Zhou, Z.; et al. Comparison of the abundance of microplastics between rural and urban areas: A case study from East Dongting Lake. Chemosphere 2019, 244, 125486. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.; Su, L.; Kellar, C.; Craig, N.J.; Keough, M.J.; Pettigrove, V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ. Pollut. 2020, 259, 113865. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Li, L.; Geng, S.; Wu, C.; Song, K.; Sun, F.; Visvanathan, C.; Xie, F.; Wang, Q. Microplastics contamination in different trophic state lakes along the middle and lower reaches of Yangtze River Basin. Environ. Pollut. 2019, 254, 112951. [Google Scholar] [CrossRef]
- Unice, K.M.; Weeber, M.P.; Abramson, M.M.; Reid, R.C.D.; van Gils, J.A.G.; Markus, A.A.; Vethaak, A.D.; Panko, J.M. Characterizing export of land-based microplastics to the estuary—Part I: Application of integrated geospatial microplastic transport models to assess tire and road wear particles in the Seine watershed. Sci. Total Environ. 2019, 646, 1639–1649. [Google Scholar] [CrossRef]
- Peller, J.; Iceman, C.; Eberhardt, L.; Clark, R.; Kostelnik, E.; Nelson, C. Tracking the distribution of microfiber pollution in a southern Lake Michigan watershed through the analysis of water, sediment and air. Environ. Sci. Processes Impacts 2019, 21, 1549–1559. [Google Scholar] [CrossRef]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Roch, S.; Walter, T.; Ittner, L.D.; Friedrich, C.; Brinker, A. A systematic study of the microplastic burden in freshwater fishes of south-western Germany - Are we searching at the right scale? Sci. Total Environ. 2019, 689, 1001–1011. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; Scott, D.T.; Seitmaganbetova, G.; van Pelt Frank, N.A.M.; John, O.H.; Jansen, M.A.K. Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci. Total Environ. 2019, 689, 413–421. [Google Scholar] [CrossRef]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Hanke, G.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.C.; van Franeker, J.; Vlachogianni, T.; et al. Guidance on Monitoring of Marine Litter in European Seas; Publications Office of the European Union: Luxembourg, 2013; 128p. [Google Scholar]
- Hendrickson, E.; Minor, E.C.; Schreiner, K. Microplastic abundance and composition in western Lake Superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018, 52, 1787–1796. [Google Scholar] [CrossRef]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef]
- Sruthy, S.; Ramasamy, E.V. Microplastic pollution in Vembanad Lake, Kerala, India: The first report of microplastics in lake and estuarine sediments in India. Environ. Pollut. 2017, 222, 315–322. [Google Scholar] [CrossRef]
- Mason, S.A.; Kammin, L.; Eriksen, M.; Aleid, G.; Wilson, S.; Box, C.; Williamson, N.; Riley, A. Pelagic plastic pollution within the surface waters of Lake Michigan, USA. J. Great Lakes Res. 2016, 42, 753–759. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Garello, N.; Ginon, L.; Abrial, E.; Espinola, L.A.; Wantzen, K.M. Massive plastic pollution in a mega-river of a developing country: Sediment deposition and ingestion by fish (Prochilodus lineatus). Environ. Pollut. 2019, 255, 113348. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Uurasjärvi, E.; Hartikainen, S.; Setälä, O.; Lehtiniemi, M.; Koistinen, A. Microplastic concentrations, size distribution, and polymer types in the surface waters of a northern European lake. Water Environ. Res. 2020, 92, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kentin, E.; Kaarto, H. An EU ban on microplastics in cosmetic products and the right to regulate. RECIEL 2018, 27, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Jâms, I.B.; Windsor, F.M.; Poudevigne-Durance, T.; Ormerod, S.J.; Durance, I. Estimating the size distribution of plastics ingested by animals. Nat. Commun. 2020, 11, 1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PlasticsEurope 2019: Plastics—the Facts 2019. An analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/it/resources/publications/1804-plastics-facts-2019 (accessed on 17 June 2020).
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef] [PubMed]
- van Wijnen, J.; Ragas, A.M.J.; Kroeze, C. Modelling global river export of microplastics to the marine environment: Sources and future trends. Sci. Total Environ. 2019, 673, 392–401. [Google Scholar] [CrossRef]
- Battisti, C.; Bazzichetto, M.; Poeta, G.; Pietrelli, L.; Acosta, A.T.R. Measuring non-biological diversity using commonly used metrics: Strengths, weaknesses and caveats for their application in beach litter management. J. Coast. Conserv. 2017, 21, 303–310. [Google Scholar] [CrossRef]
- González, D.; Hanke, G.; Tweehuysen, G.; Bellert, B.; Holzhauer, M.; Palatinus, A.; Hohenblum, P.; Oosterbaan, L. Riverine Litter Monitoring—Options and Recommendations. MSFD GES TG Marine Litter Thematic Report; JRC Technical Report 2016; EUR 28307; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]










| Matrix | Type of Water Body | Number of Sampled Water Bodies | Mean Value | Range across Water Bodies | Region Represented |
|---|---|---|---|---|---|
| Water | River | 168 | 11,128 items/m3 | 0–510,140 | Am, As, Eu, Oc |
| 12 | 591.7 items/L | 0.1–2083.5 | Af, Am, As, Oc | ||
| 10 | 4,087,325 items/km2 | 17,127,500–40,873,250 | As | ||
| 4 | 0.019 g/m3 | 0.005–0.034 | As | ||
| 2 | 10.2 items/kg | 6.8–13.6 | As | ||
| 1 | 0.638 items/m2 | one water body | As | ||
| Lake | 62 | 2561.959 items/m3 | 0.0005–8925 | As, Eu | |
| 18 | 92,032 items/km2 | 2779–400,500 | Am, As, Eu | ||
| 10 | 12.2 items/L | 0.8–21.7 | Am, As | ||
| 1 | 1200 mg/km2 | one water body | Am | ||
| 1 | 0.407 items/m2 | one water body | Af | ||
| Sediment (beaches and nearshore inclusive) | River | 96 | 1161.452 items/kg | 0.0000303–32,947 | Af, Am, As, Eu, Oc |
| 5 | 4835 items/m2 | 5–13,759 | Am, As | ||
| 2 | 0.18983 items/g | 0.16665–0.213 | Am, As | ||
| 1 | 87 items | one water body | Am | ||
| 1 | 223 items/L | one water body | Am | ||
| 1 | 0.00077 items/m3 | one water body | As | ||
| Lake | 47 | 525.0905 items/kg | 0.2733–13,925 | Af, Am, As, Eu | |
| 14 | 891 items/m2 | 17–3508 | Am, As, Eu | ||
| 3 | 29.8 g | 12.3–42.1 | Eu | ||
| 1 | 35 items | one water body | Am | ||
| 1 | 2.9 items/cm3 | one water body | Eu | ||
| 1 | 537.5 items/m3 | one water body | As | ||
| 1 | 4 items/L | one water body | Eu | ||
| 1 | 0.24 mg/g | one water body | Eu | ||
| 1 | 2680.5 g/m2 | one water body | As |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cera, A.; Cesarini, G.; Scalici, M. Microplastics in Freshwater: What Is the News from the World? Diversity 2020, 12, 276. https://doi.org/10.3390/d12070276
Cera A, Cesarini G, Scalici M. Microplastics in Freshwater: What Is the News from the World? Diversity. 2020; 12(7):276. https://doi.org/10.3390/d12070276
Chicago/Turabian StyleCera, Alessandra, Giulia Cesarini, and Massimiliano Scalici. 2020. "Microplastics in Freshwater: What Is the News from the World?" Diversity 12, no. 7: 276. https://doi.org/10.3390/d12070276
APA StyleCera, A., Cesarini, G., & Scalici, M. (2020). Microplastics in Freshwater: What Is the News from the World? Diversity, 12(7), 276. https://doi.org/10.3390/d12070276