Highly Productive Tropical Seagrass Beds Support Diverse Consumers and a Large Organic Carbon Pool in the Sediments
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Site
2.2. Ecopath Modeling
2.3. Model Compartment Sampling
2.3.1. Fish (Compartments 1–10)
2.3.2. Sea Turtle (Compartments 11–12)
2.3.3. Macroinvertebrates (Compartments 13–18)
2.3.4. Cnidarians (Compartment 19)
2.3.5. Zooplankton (Compartment 20)
2.3.6. Primary Producers (Compartments 21–23)
2.3.7. Detritus (Compartment 24)
2.4. Model Balancing and Verification
2.5. Ecosystem Parameter Analysis
3. Result
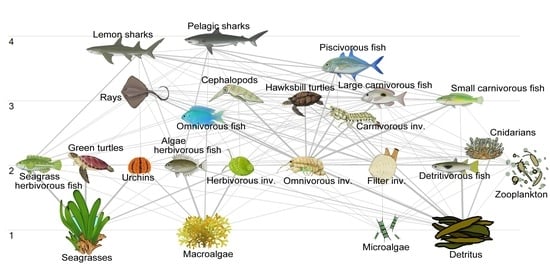
3.1. Trophic Flows and Networks
3.2. Keystone Species and Mixed Trophic Impacts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Chiscano, C.L. Seagrass biomass and production: A reassessment. Aquat. Bot. 1999, 65, 159–174. [Google Scholar] [CrossRef]
- Duarte, C.M.; Marbà, N.; Gacia, E.; Fourqurean, J.; Beggins, J.; Barrón, C.; Apostolaki, E. Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef] [Green Version]
- Jernakoff, P.; Nielsen, J. Plant-animal associations in two species of seagrasses in Western Australia. Aquat. Bot. 1998, 60, 359–376. [Google Scholar] [CrossRef]
- Hyndes, G.A.; Lavery, P.S. Does transported seagrass provide an important trophic link in unvegetated, nearshore areas? Estuar. Coast. Shelf Sci. 2005, 63, 633–643. [Google Scholar] [CrossRef]
- Parrish, J. Fish communities of interacting shallow-water habitats in tropical oceanic regions. Mar. Ecol. Prog. Ser. 1989, 58, 143–160. [Google Scholar] [CrossRef]
- Duffy, J. Biodiversity and the functioning of seagrass ecosystems. Mar. Ecol. Prog. Ser. 2006, 311, 233–250. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.E. Economic Benefits of Habitat Restoration: Seagrass and the Virginia Hard-Shell Blue Crab Fishery. N. Am. J. Fish. Manag. 1989, 9, 140–149. [Google Scholar] [CrossRef]
- Thayer, G.W.; Bjorndal, K.; Ogden, J.C.; Williams, S.L.; Zieman, J.C. Role of Larger Herbivores in Seagrass Communities. Estuaries 1984, 7, 351–376. [Google Scholar] [CrossRef]
- Duarte, C.M. Seagrass nutrient content. Mar. Ecol. Prog. Ser. 1990, 67, 201–207. [Google Scholar] [CrossRef]
- Cebrian, J. Variability and control of carbon consumption, export, and accumulation in marine communities. Limnol. Oceanogr. 2002, 47, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Mateo, M.; Cebrián, J.; Dunton, K.; Mutchler, T. Carbon flux in seagrass ecosystems. In Seagrasses: Biology, Ecology, and Conservation; Larkum, A.W., Orth, R.J., Duarte, C., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 159–192. [Google Scholar]
- Huang, Y.-H.; Lee, C.-L.; Chung, C.-Y.; Hsiao, S.-C.; Lin, H.-J. Carbon budgets of multispecies seagrass beds at Dongsha Island in the South China Sea. Mar. Environ. Res. 2015, 106, 92–102. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wen, C.K.; Huang, Y.-H.; Chung, C.-Y.; Lin, H.-J. Ontogenetic Habitat Usage of Juvenile Carnivorous Fish Among Seagrass-Coral Mosaic Habitats. Diversity 2019, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.J.; Hsiao, S.C. Phenology and Production of Seagrass and Macroalgae of Dongsha Island; Research Report; Marine National Park Headquarters: Kaohsiung, Taiwan, 2010.
- Lin, H.J.; Hsiao, S.C. Biota Researches and Keystone Assessment of Dongsha Island; Research Report; Marine National Park Headquarters: Kaohsiung, Taiwan, 2011.
- Lee, C.-L.; Huang, Y.-H.; Chung, C.-Y.; Lin, H.-J. Tidal variation in fish assemblages and trophic structures in tropical Indo-Pacific seagrass beds. Zool. Stud. 2014, 53, 56. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, S.E.; Chon, T.S.; Recknagel, F. Handbook of Ecological Modelling and Informatics; Wit Press: Southampton, UK, 2009. [Google Scholar]
- Lin, H.-J.; Dai, X.-X.; Shao, K.-T.; Su, H.-M.; Lo, W.-T.; Hsieh, H.-L.; Fang, L.-S.; Hung, J.-J. Trophic structure and functioning in a eutrophic and poorly flushed lagoon in southwestern Taiwan. Mar. Environ. Res. 2006, 62, 61–82. [Google Scholar] [CrossRef]
- Liu, P.-J.; Shao, K.-T.; Jan, R.-Q.; Fan, T.-Y.; Wong, S.-L.; Hwang, J.-S.; Chen, J.-P.; Chen, C.-C.; Lin, H.-J. A trophic model of fringing coral reefs in Nanwan Bay, southern Taiwan suggests overfishing. Mar. Environ. Res. 2009, 68, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.J.; Hsieh, L.Y.; Liu, P.J. Seagrasses of Tongsha Island, with descriptions of four new records to Taiwan. Bot. Bull. Acad. Sin. 2005, 46, 163–168. [Google Scholar]
- Christensen, V.; Walters, C.J.; Pauly, D.; Forrest, R. Ecopath with Ecosim Version 6: User’s Guide; University of British Columbia, Fisheries Centre: Vancouver, BC, Canada, 2008. [Google Scholar]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. 2012. Available online: www.fishbase.org (accessed on 22 September 2021).
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Palomares, M.; Pauly, D. A multiple regression model for prediction the food consumption of Marine Fish populations. Mar. Freshw. Res. 1989, 40, 259–273. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Pauly, D. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature and salinity. Mar. Freshw. Res. 1998, 49, 447–453. [Google Scholar] [CrossRef]
- Wabnitz, C.C.C. Sea Turtle Conservation and Ecosystem-Based Management with a Focus on Green Turtles (Chelonia mydas) and Seagrass Beds. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2010. [Google Scholar]
- Bjorndal, K.A. Foraging ecology and nutrition of sea turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: New York, NY, USA, 1997; pp. 199–231. [Google Scholar]
- Bjorndal, K.; Bolten, A.; Chaloupka, M. Survival probability estimates for immature green turtles Chelonia mydas in the Bahamas. Mar. Ecol. Prog. Ser. 2003, 252, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Crouse, D. Population modeling and implications for Caribbean hawksbill sea turtle management. Chelonian Conserv. Biol. 1999, 3, 185–188. [Google Scholar]
- Brand, S.J.; Lanyon, J.M.; Limpus, C.J. Digesta composition and retention times in wild immature green turtles, Chelonia mydas: A preliminary investigation. Mar. Freshw. Res. 1999, 50, 145–147. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clark, K.R. Comparing the severity of disturbance: A meta-analysis of marine macrobenthic community data. Mar. Ecol. Prog. Ser. 1993, 92, 221–231. [Google Scholar] [CrossRef]
- Arana, H.H.; Warwick, R.; Attrill, M.; Rowden, A.; Gold-Bouchot, G. Assessing the impact of oil-related activities on benthic macroinfauna assemblages of the Campeche shelf, Southern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2005, 289, 89–107. [Google Scholar] [CrossRef] [Green Version]
- Balgos, M.C.; Pauly, D. Age and growth of the squid Sepioteuthis lessoniana in NW Luzon, Philippines. Afr. J. Mar. Sci. 1998, 20, 449–452. [Google Scholar] [CrossRef]
- Chávez, E.; Garduño, M.; Arreguín-Sánchez, F. Trophic dynamic structure of Celestun Lagoon, Southern Gulf of Mexico. In Trophic Models of Aquatic Ecosystems; Christensen, V., Pauly, D., Eds.; International Center for Living Aquatic Resources Management Conference Proceedings: Manila, Phillipines, 1993; pp. 186–192. [Google Scholar]
- Opitz, S. Trophic Interactions in Caribbean Coral Reefs; ICLARM Technical Report; International Center for Living Aquatic Resources Management: Manila, Phillipines, 1996; p. 43. [Google Scholar]
- Chang, W.B.; Lei, C.H. The development and energy content of a brackishwater copepod, Apocyclops royi (Lindberg), reared in the laboratory. Bull. Inst. Zool. Acad. Sin. 1993, 32, 62–81. [Google Scholar]
- Pauly, D.; Christensen, V. Stratified models of large marine ecosystems: A general approach and an application to the South China Sea. In Large Marine Ecosystems: Stress, Mitigation and Sustainability; Sherman, K., Alexander, L.M., Gold, B.D., Eds.; AAAS Press: Washington, DC, USA, 1993; pp. 148–174. [Google Scholar]
- Zieman, J.C. Methods for the study of the growth and production of turtle grass, Thalassia testudinum König. Aquaculture 1974, 4, 139–143. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining hlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Chen, C.C.; Shiah, F.K.; Jan, S.; Hsu, S.S. The Ecosystem Research of Donsha Atoll; Research Report; Marine National Park Headquarters: Kaohsiung, Taiwan, 2011.
- Migné, A.; Davoult, D.; Splimont, N.; Menu, D.; Boucher, G.; Gattuso, J.P.; Rybarczyk, H. A closed-chamber CO2-flux method for estimating intertidal primary production and respiration under emersed conditions. Mar. Biol. 2002, 140, 865–869. [Google Scholar]
- Lee, L.; Hsieh, L.; Lin, H.-J. In situ production and respiration of the benthic community during emersion on subtropical intertidal sandflats. Mar. Ecol. Prog. Ser. 2011, 441, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.F. Measurement of organic carbon in seawater. Limnol. Oceanogr. 1961, 6, 259–261. [Google Scholar] [CrossRef]
- Shiah, F.K.; Chung, S.W.; Kao, S.J.; Gong, G.C.; Liu, K.K. Biological and hydrographical responses to tropical cyclones (typhoons) in the continental shelf of the Taiwan Strait. Cont. Shelf Res. 2000, 20, 2029–2044. [Google Scholar] [CrossRef]
- Sobczak, W.V.; Cloern, J.E.; Jassby, A.D.; Müller-Solger, A.B. Bioavailability of organic matter in a highly distributed estuary: The role of detrital and algal resources. Proc. Natl. Acad. Sci. USA 2002, 99, 8101–8105. [Google Scholar] [CrossRef] [Green Version]
- Majkowski, J. Usefulness and applicability of sensitivity analysis in a multispecies approach to fisheries management. In Theory and Management of Tropical Fisheries; Pauly, D., Murphy, G.I., Eds.; International Center for Living Aquatic Resources Management Conference Proceedings: Manila, Phillipines, 1982; Volume 9, pp. 149–165. [Google Scholar]
- Tennenbaum, S.; Ulanowicz, R.E. Growth and Development: Ecosystems Phenomenology. Estuaries 1988, 11, 73. [Google Scholar] [CrossRef]
- Wulff, F.; Field, J.G.; Mann, K.H. Network Analysis in Marine Ecology-Methods And Applications-Coastal And Estuarine Studies Formerly Lecture Notes on Coastal And Estuarine Studies; Springer: New York, NY, USA, 1989. [Google Scholar]
- Fath, B.D.; Patten, B.C. Review of the Foundations of Network Environ Analysis. Ecosystems 1999, 2, 167–179. [Google Scholar] [CrossRef]
- Christian, R.R.; Baird, D.; Luczkovich, J.; Johnson, J.C.; Scharler, U.M.; Ulanowicz, R.E. Role of network analysis in comparative ecosystem ecology of estuaries. In Aquatic Food Webs: An Ecosystem Approach; Belgrano, A., Scharler, U.M., Dunne, J., Ulanowicz, R.E., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 25–40. [Google Scholar]
- Odum, E.P. The strategy of ecosystem development. Science 2014, 104, 203–216. [Google Scholar] [CrossRef]
- Kay, J.J.; Graham, L.A.; Ulanowicz, R.E. A detailed guide to network analysis. In Network Analysis in Marine Ecology: Methods and Applications; Wulff, F., Field, J.G., Mann, K.H., Eds.; Springer: New York, NY, USA, 1989; pp. 15–61. [Google Scholar]
- Ulanowicz, R.E.; Norden, J.S. Symmetrical overhead in flow networks. Int. J. Syst. Sci. 1990, 21, 429–437. [Google Scholar] [CrossRef]
- Libralato, S.; Christensen, V.; Pauly, D. A method for identifying keystone species in food web models. Ecol. Model. 2006, 195, 153–171. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Puccia, C.J. Mixed trophic impacts in ecosystems. Coenoses 1990, 5, 7–16. [Google Scholar]
- Lee, C.; Huang, Y.; Chung, C.; Hsiao, S.; Lin, H. Herbivory in multi-species, tropical seagrass beds. Mar. Ecol. Prog. Ser. 2015, 525, 65–80. [Google Scholar] [CrossRef]
- Nakamoto, K.; Hayakawa, J.; Kawamura, T.; Ohtsuchi, N.; Yamada, H.; Kitagawa, T.; Watanabe, Y. Seasonal fluctuation in food sources of herbivorous gastropods in a subtropical seagrass bed estimated by stable isotope analysis. J. Mar. Biol. Assoc. UK 2018, 99, 1119–1125. [Google Scholar] [CrossRef]
- Du, J.; Zheng, X.; Peristiwady, T.; Liao, J.; Makatipu, P.C.; Yin, X.; Hu, W.; Koagouw, W.; Chen, B. Food sources and trophic structure of fishes and benthic macroinvertebrates in a tropical seagrass meadow revealed by stable isotope analysis. Mar. Biol. Res. 2016, 12, 748–757. [Google Scholar] [CrossRef]
- Vonk, J.A.; Christianen, M.J.; Stapel, J. Redefining the trophic importance of seagrasses for fauna in tropical Indo-Pacific meadows. Estuarine Coast. Shelf Sci. 2008, 79, 653–660. [Google Scholar] [CrossRef]
- Manickchand-Heileman, S.; Arreguín-Sánchez, F.; Lara-Dominguez, A.; Soto, L.A. Energy flow and network analysis of Terminos Lagoon, SW Gulf of Mexico. J. Fish Biol. 1998, 53, 179–197. [Google Scholar] [CrossRef]
- Riddle, M.J.; Alongi, M.D.; Dayton, K.P.; Hansen, A.J.; Klumpp, W.D. Detritical pathways in a coral reef lagoon: I. Macrofaunal biomass and estimates of production. Mar. Biol. 1990, 104, 109–118. [Google Scholar]
- Sorokin, I.Y. Aspects of trophic relations, productivity and energy balance in coral-reef ecosystems. In Ecosystems of the World: Coral Reefs; Dubinski, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 25, pp. 401–409. [Google Scholar]
- Romero, J.; Pérez, M.; Mateo, M.; Sala, E. The belowground organs of the Mediterranean seagrass Posidonia oceanica as a biogeochemical sink. Aquat. Bot. 1994, 47, 13–19. [Google Scholar] [CrossRef]
- Mateo, M.; Romero, J.; Pérez, M.; Littler, M.; Littler, D. Dynamics of Millenary Organic Deposits Resulting from the Growth of the Mediterranean Seagrass Posidonia oceanica. Estuarine Coast. Shelf Sci. 1997, 44, 103–110. [Google Scholar] [CrossRef]
- Peduzzi, P.; Herndl, G.J. Decomposition and significance of seagrassleaf litter (Cymodocea nodosa) for the microbial food web in coastal waters (Gulf of Trieste, Northern Adriatic Sea). Mar. Ecol. Prog. Ser. 1991, 71, 163–174. [Google Scholar] [CrossRef]
- Robertson, A.; Mann, K.H. The role of isopods and amphipods in the initial fragmentation of eelgrass detritus in Nova Scotia, Canada. Mar. Biol. 1980, 59, 63–69. [Google Scholar] [CrossRef]
- Bologna, P.A. Assessing within habitat variability in plant demography, faunal density, and secondary production in an eelgrass (Zostera marina L.) bed. J. Exp. Mar. Biol. Ecol. 2006, 329, 122–134. [Google Scholar] [CrossRef]
- Arias-González, J.E.; Delesalle, B.; Salvat, B.; Galzin, R. Torphic functioning of the Tiahura reef sector, Moorea Island, French Polynesia. Coral Reefs 1997, 16, 231–246. [Google Scholar]
- Johnson, C.; Klumpp, D.; Field, J.; Bradbury, R. Carbon flux on coral reefs: Effects of large shifts in community structure. Mar. Ecol. Prog. Ser. 1995, 126, 123–143. [Google Scholar] [CrossRef] [Green Version]
- Paine, R.T. A Note on Trophic Complexity and Community Stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Krivan, V.; Ovadia, O. Trophic cascades: The primacy of trait-mediated indirect interactions. Ecol. Lett. 2004, 7, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.C. Distribution Heterogeneity of the Rays Pateobatis fai and Aetobatus ocellatus in Shallow Water of Dongsha Island-Hypothesis Testing. Master’s Thesis, National Sun Yat-sen University, Kaohsiung, Taiwan, 2019. [Google Scholar]
- DeAngelis, B.; McCandless, C.; Kohler, N.; Recksiek, C.; Skomal, G. First characterization of shark nursery habitat in the United States Virgin Islands: Evidence of habitat partitioning by two shark species. Mar. Ecol. Prog. Ser. 2008, 358, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Legare, B.; Kneebone, J.; de Angelis, B.; Skomal, G. The spatiotemporal dynamics of habitat use by blacktip (Carcharhinus limbatus) and lemon (Negaprion brevirostris) sharks in nurseries of St. John, United States Virgin Islands. Mar. Biol. 2015, 162, 699–716. [Google Scholar] [CrossRef]
- Legare, B.; Skomal, G.; DeAngelis, B. Diel movements of the blacktip shark (Carcharhinus limbatus) in a Caribbean nursery. Environ. Boil. Fishes 2018, 101, 1011–1023. [Google Scholar] [CrossRef]
- Heithaus, M.R.; Dill, L.; Marshall, G.; Buhleier, B. Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar. Biol. 2002, 140, 237–248. [Google Scholar]
- Heithaus, M.R.; Vaudo, J.J.; Kreicker, S.; Layman, C.A.; Krützen, M.; Burkholder, D.A.; Gastrich, K.; Bessey, C.; Sarabia, R.; Cameron, K.; et al. Apparent resource partitioning and trophic structure of large-bodied marine predators in a relatively pristine seagrass ecosystem. Mar. Ecol. Prog. Ser. 2013, 481, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Jackson, E.L.; Rees, S.; Wilding, C.; Attrill, M.J. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conserv. Biol. 2015, 29, 899–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.-F.; Chen, K.-Y.; Lin, H.-J. Significance of belowground production to the long-term carbon sequestration of intertidal seagrass beds. Sci. Total Environ. 2021, 800, 149579. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987–996. [Google Scholar] [CrossRef] [Green Version]




| Basic Input | Calculated Output | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | B | P/B | Q/B | EE | Trophic Level | Net Efficiency | Omnivory Index | Flow to Detritus | Keystone Index | Relative Total Impact | |
| 1 | Pelagic sharks | 0.23 | 0.32 | 3.90 | (0.00) | 3.82 | 0.10 | 0.35 | 0.25 | 0.02 | 0.78 |
| 2 | Lemon sharks | 3.25 | 0.31 | 2.60 | (0.01) | 3.74 | 0.15 | 0.37 | 2.69 | 0.06 | 0.86 |
| 3 | Rays | 3.86 | 0.63 | 3.30 | (0.00) | 3.17 | 0.24 | 0.07 | 4.97 | −0.20 | 0.47 |
| 4 | Piscivorous fish | 12.46 | 0.64 | 5.49 | (0.28) | 3.49 | 0.14 | 0.25 | 19.38 | 0.13 | 1.00 |
| 5 | Large carnivorous fish | 52.44 | 1.55 | 8.08 | (0.26) | 3.04 | 0.24 | 0.02 | 144.99 | −0.09 | 0.61 |
| 6 | Small carnivorous fish | 13.69 | 1.61 | 12.59 | (0.83) | 3.01 | 0.16 | 0.02 | 38.14 | −0.51 | 0.23 |
| 7 | Omnivorous fish | 2.60 | 2.27 | 19.79 | (0.58) | 2.79 | 0.14 | 0.21 | 12.74 | −0.66 | 0.17 |
| 8 | Detritivorous fish | 2.79 | 1.60 | 17.42 | (0.71) | 2.00 | 0.11 | 0.00 | 11.00 | −1.46 | 0.03 |
| 9 | Seagrass herbivorous fish | 15.06 | 1.79 | 29.94 | (0.38) | 2.00 | 0.07 | 0.00 | 107.02 | −0.16 | 0.52 |
| 10 | Algae herbivorous fish | 6.13 | 4.39 | 24.75 | (0.34) | 2.00 | 0.22 | 0.00 | 48.02 | −0.47 | 0.25 |
| 11 | Hawksbill turtles | 0.00 | 0.10 | 3.50 | (0.22) | 2.97 | 0.04 | 0.09 | 0.00 | −3.65 | 0.00 |
| 12 | Green turtles | 0.01 | 0.11 | 6.76 | (0.08) | 2.00 | 0.02 | 0.00 | 0.01 | −3.84 | 0.00 |
| 13 | Cephalopods | 0.12 | 3.10 | (15.50) | (0.48) | 3.07 | 0.25 | 0.09 | 0.56 | −2.70 | 0.00 |
| 14 | Urchins | 2.92 | 1.54 | (7.70) | (0.41) | 2.00 | 0.25 | 0.00 | 7.13 | −1.01 | 0.07 |
| 15 | Carnivorous invertebrates | 5.55 | 8.27 | (41.36) | (0.16) | 2.82 | 0.25 | 0.17 | 84.27 | −0.65 | 0.17 |
| 16 | Omnivorous invertebrates | 145.35 | 13.48 | (67.41) | (0.47) | 2.02 | 0.25 | 0.02 | 3195.91 | 0.07 | 0.93 |
| 17 | Filter invertebrates | 43.92 | 8.21 | (41.05) | (0.82) | 2.01 | 0.25 | 0.01 | 426.08 | 0.02 | 0.80 |
| 18 | Herbivorous invertebrates | 4.28 | 6.80 | (34.00) | (0.26) | 2.00 | 0.25 | 0.00 | 50.67 | −0.55 | 0.21 |
| 19 | Cnidarians | 6.12 | 2.09 | (10.45) | (0.40) | 2.15 | 0.25 | 0.24 | 19.20 | −1.20 | 0.05 |
| 20 | Zooplankton | 0.60 | 32.00 | 192.00 | (0.81) | 2.01 | 0.21 | 0.12 | 26.77 | −1.34 | 0.03 |
| 21 | Macroalgae | 124.54 | 19.70 | (0.16) | 1.00 | 0.00 | 2070.21 | −0.15 | 0.56 | ||
| 22 | Microalgae | 1.42 | 26.55 | (0.71) | 1.00 | 0.00 | 10.99 | −1.82 | 0.01 | ||
| 23 | Seagrasses | 1836.90 | 37.18 | (0.01) | 1.00 | 0.00 | 67748.51 | −0.79 | 0.62 | ||
| 24 | Detritus | 30.31 | (0.15) | 1.00 | 0.00 | 0.07 | 0.00 | ||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pelagic sharks | <0.001 | |||||||||||||||||||
| 2 | Lemon sharks | 0.010 | |||||||||||||||||||
| 3 | Rays | 0.010 | <0.001 | ||||||||||||||||||
| 4 | Piscivorous fish | 0.254 | 0.221 | 0.001 | 0.001 | 0.001 | <0.001 | ||||||||||||||
| 5 | Large carnivorous fish | 0.355 | 0.387 | 0.001 | 0.249 | 0.001 | 0.001 | <0.001 | |||||||||||||
| 6 | Small carnivorous fish | <0.001 | 0.010 | 0.186 | 0.013 | 0.002 | 0.001 | ||||||||||||||
| 7 | Omnivorous fish | 0.050 | 0.005 | <0.001 | 0.039 | 0.001 | 0.002 | 0.001 | |||||||||||||
| 8 | Detritivorous fish | <0.001 | 0.025 | 0.001 | 0.036 | 0.001 | 0.002 | 0.001 | |||||||||||||
| 9 | Seagrass herbivorous fish | 0.110 | 0.240 | 0.001 | 0.103 | 0.002 | 0.002 | 0.001 | |||||||||||||
| 10 | Algae herbivorous fish | 0.210 | 0.121 | 0.001 | 0.103 | 0.002 | 0.002 | 0.001 | |||||||||||||
| 11 | Hawksbill turtles | <0.001 | |||||||||||||||||||
| 12 | Green turtles | <0.001 | |||||||||||||||||||
| 13 | Cephalopods | <0.001 | 0.001 | <0.001 | 0.001 | <0.001 | 0.050 | ||||||||||||||
| 14 | Urchins | 0.100 | 0.001 | 0.001 | 0.001 | ||||||||||||||||
| 15 | Carnivorous invertebrates | 0.106 | 0.002 | 0.010 | 0.005 | 0.015 | 0.010 | 0.001 | 0.001 | ||||||||||||
| 16 | Omnivorous invertebrates | 0.415 | 0.281 | 0.868 | 0.964 | 0.557 | 0.440 | 0.829 | 0.545 | 0.001 | |||||||||||
| 17 | Filter invertebrates | 0.309 | 0.100 | 0.009 | 0.155 | 0.250 | 0.009 | 0.245 | 0.018 | 0.050 | 0.005 | ||||||||||
| 18 | Herbivorous invertebrates | 0.055 | <0.001 | 0.001 | 0.003 | 0.001 | 0.100 | 0.001 | 0.001 | 0.005 | 0.001 | ||||||||||
| 19 | Cnidarians | 0.025 | 0.001 | 0.050 | 0.001 | 0.001 | 0.001 | ||||||||||||||
| 20 | Zooplankton | <0.001 | 0.002 | 0.002 | 0.002 | 0.001 | <0.001 | <0.001 | 0.007 | 0.010 | 0.005 | ||||||||||
| 21 | Macroalgae | 0.224 | 0.031 | 0.022 | 0.856 | 0.050 | 0.048 | 0.250 | 0.013 | 0.690 | |||||||||||
| 22 | Microalgae | 0.001 | 0.003 | 0.001 | 0.011 | <0.001 | 0.010 | 0.010 | |||||||||||||
| 23 | Seagrasses | 0.974 | 0.005 | 0.951 | 0.700 | 0.005 | 0.293 | ||||||||||||||
| 24 | Detritus | 0.001 | 0.018 | 0.020 | 0.964 | 0.004 | 0.138 | 0.050 | 0.200 | 0.961 | 0.972 | 0.017 | 0.330 | 0.100 | |||||||
| Input from outside | 0.100 | 0.100 | 0.010 | 0.600 | 0.880 |
| Parameter | Abbreviation | Description | Unit |
|---|---|---|---|
| Ascendency | A | A measure of the magnitude of organic matter flowing through a food web | % |
| Detritivory/herbivory ratio | D/H | A measure of the importance of detritivory flows relative to herbivory flows in a system | |
| Finn cycling index | FCI | A measure of the relative importance of cycling to the total system throughput (TST) in a system | % |
| Net primary production/total biomass ratio | P/B | The relationship between net primary production and total biomass in a system | |
| Net primary production/respiration ratio | P/R | The relationship between net primary production and respiration in a system | |
| Omnivory index | A measure of the distribution of feeding interactions among trophic levels in a system | ||
| Total biomass/TST ratio | B/TST | The amount of biomass necessary to maintain one unit of flow in a system | |
| Total system throughput | TST | Sum of all flows in a system; a measure of system activity | g WW m−2 yr−1 |
| Trophic transfer efficiency | TE | A measure of the fraction of the input of organic matter to a given level that was transferred to the next higher level | % |
| Species | Family | Density (per ha) | Body-Length Range (cm) | Diet | Major Habitat | Functional Group |
|---|---|---|---|---|---|---|
| Tiger shark (Galeocerdo cuvier) | Carcharhinidae | <0.01 | 220–370 | Fish, shark, ray, sea turtle * | Seagrass bed, Reef | pelagic shark |
| Black tip shark (Carcharhinus limbatus) | Carcharhinidae | 1.29 | 90–200 | Fish (mostly carangid, lethrinid lutjanid) | Reef | pelagic shark |
| Sharptooth lemon shark (Negaprion acutidens) | Carcharhinidae | 23.01 | 60–200 | Fish (mostly mugilid, carangid, lethrinid lutjanid, scarid) Squid (Sepioteuthis lessoniana) | Seagrass bed, Inner lagoon, Reef | lemon shark |
| Pink whip ray (Pateobatis fai) | Dasyatidae | 12.11 | 60–150 | Crab, Shrimp, Clam, Snail, Urchin | Seagrass bed, Inner lagoon | ray |
| Round ribbon-tail ray (Taeniura meyeni) | Dasyatidae | 1.60 | 100–200 | Crab, Shrimp, Clam, Urchin | Seagrass bed, Reef | ray |
| Ocellated eagle ray (Aetobatus ocellatus) | Aetobatidae | 8.09 | 50–160 | Crab, Clam, Snail | Seagrass bed, Reef | ray |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Lin, W.-J.; Liu, P.-J.; Shao, K.-T.; Lin, H.-J. Highly Productive Tropical Seagrass Beds Support Diverse Consumers and a Large Organic Carbon Pool in the Sediments. Diversity 2021, 13, 544. https://doi.org/10.3390/d13110544
Lee C-L, Lin W-J, Liu P-J, Shao K-T, Lin H-J. Highly Productive Tropical Seagrass Beds Support Diverse Consumers and a Large Organic Carbon Pool in the Sediments. Diversity. 2021; 13(11):544. https://doi.org/10.3390/d13110544
Chicago/Turabian StyleLee, Chen-Lu, Wei-Jen Lin, Pi-Jen Liu, Kwang-Tsao Shao, and Hsing-Juh Lin. 2021. "Highly Productive Tropical Seagrass Beds Support Diverse Consumers and a Large Organic Carbon Pool in the Sediments" Diversity 13, no. 11: 544. https://doi.org/10.3390/d13110544
APA StyleLee, C. -L., Lin, W. -J., Liu, P. -J., Shao, K. -T., & Lin, H. -J. (2021). Highly Productive Tropical Seagrass Beds Support Diverse Consumers and a Large Organic Carbon Pool in the Sediments. Diversity, 13(11), 544. https://doi.org/10.3390/d13110544