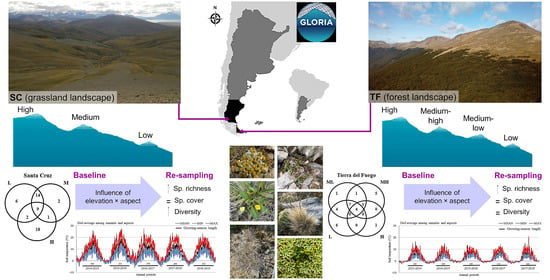
Variation in Alpine Plant Diversity and Soil Temperatures in Two Mountain Landscapes of South Patagonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling Methodology
2.3. Data and Statistical Analyses
3. Results
3.1. Plant Variation
3.2. Soil Temperature Variation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Species | Code | LF | Origin | Elevation | L-SC | M-SC | H-SC | L-TF | ML-TF | MH-TF | H-TF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m a.s.l.) | BL | RS | BL | RS | BL | RS | BL | RS | BL | RS | BL | RS | BL | RS | |||||
| Alstroemeriaceae | Alstroemeria patagonica Phil. 1896 | ALPA | EH | N | 0–1300 | 19 | 31 | ||||||||||||
| Amaryllidaceae | Tristagma nivale Poepp. 1833 | TRNI | EH | N | 300–3200 | 38 | 44 | ||||||||||||
| Apiaceae | Azorella 01 | AZO01 | CU | N | - | 6 | |||||||||||||
| Apiaceae | Azorella lycopodioides Gaudich. 1825 | AZLY | CU | N | 0–2500 | 25 | 38 | 38 | 38 | 13 | 13 | ||||||||
| Apiaceae | Azorella monantha Clos 1848 | AZMO | CU | N | 0–3000 | 19 | 25 | 25 | 25 | 50 | 50 | ||||||||
| Apiaceae | Azorella prolifera (Cav.) G.M. Plunkett & A.N. Nicolas 2017 | AZPR | S | N | 0–1500 | 6 | 6 | ||||||||||||
| Apiaceae | Azorella ranunculus d’Urv. 1826 | AZRA | PH | N | 0–900 | 56 | |||||||||||||
| Apiaceae | Azorella selago Hook. f. 1847 | AZSE | CU | N | 0–1000 | 25 | 6 | 19 | 31 | 25 | 19 | 6 | 6 | ||||||
| Apiaceae | Bolax gummifera (Lam.) Spreng. 1818 | BOGU | CU | NE | 0–3500 | 88 | 94 | 75 | 75 | 19 | 19 | ||||||||
| Asteraceae | Abrotanella emarginata (Gaudich.) Cass. 1825 | ABEM | CU | NE | 0–1000 | 69 | 75 | 69 | 81 | 50 | 44 | ||||||||
| Asteraceae | Asteraceae 01 | AST01 | EH | N | - | 13 | |||||||||||||
| Asteraceae | Asteraceae 02 | AST02 | EH | N | - | 13 | 44 | 44 | 50 | ||||||||||
| Asteraceae | Asteraceae 03 | AST03 | EH | N | - | 31 | 19 | ||||||||||||
| Asteraceae | Chiliotrichum diffusum (G. Forst.) Kuntze 1898 | CHDI | S | N | 0–2700 | 6 | 6 | ||||||||||||
| Asteraceae | Erigeron imbricatus Vierh. 1916 | ERIM | EH | N | 0–1000 | 19 | 25 | ||||||||||||
| Asteraceae | Gamochaeta spiciformis (Sch. Bip.) Cabrera 1961 | GASP | EH | N | 200–1500 | 6 | |||||||||||||
| Asteraceae | Hieracium antarcticum d’Urv. 1826 | HIAN | EH | N | 0–2000 | 25 | 25 | 6 | 19 | 6 | |||||||||
| Asteraceae | Hypochaeris incana (Hook. & Arn.) Macloskie var. incana 1906 | HYIN | EH | N | 0–1500 | 6 | |||||||||||||
| Asteraceae | Leucheria leontopodioides (Kuntze) K. Schum. 1900 | LELE | EH | N | 0–1500 | 6 | 19 | ||||||||||||
| Asteraceae | Nardophyllum bryoides (Lam.) Cabrera 1954 | NABR | CU | N | 0–1200 | 6 | 25 | 50 | 50 | 44 | 19 | ||||||||
| Asteraceae | Nassauvia 01 | NAS01 | PH | N | - | 13 | |||||||||||||
| Asteraceae | Nassauvia aculeata (Less.) Poepp. & Endl. var. azorelloides (Speg.) Cabrera 1982 | NAAC | PH | N | 0–1000 | 50 | 50 | 25 | 25 | 44 | 31 | ||||||||
| Asteraceae | Nassauvia darwinii (Hook. & Arn.) O. Hoffm. & Dusén 1901 | NADA | SS | N | 0–1000 | 13 | 38 | ||||||||||||
| Asteraceae | Nassauvia glomerulosa (Lag. ex Lindl.) D. Don 1832 | NAGL | CU | N | 0–1500 | 75 | 81 | 88 | 81 | ||||||||||
| Asteraceae | Nassauvia pygmaea (Cass.) Hook. f. var. pygmaea 1847 | NAPY | EH | N | 0–1000 | 6 | |||||||||||||
| Asteraceae | Perezia magellanica (L. f.) Lag. 1811 | PEMA | EH | N | 200–1400 | 25 | 25 | ||||||||||||
| Asteraceae | Perezia pilifera (D. Don) Hook. & Arn. 1835 | PEPI | PH | N | 0–4300 | 13 | |||||||||||||
| Asteraceae | Perezia recurvata (Vahl) Less. 1830 | PERE | EH | N | 0–1000 | 31 | 31 | 31 | 25 | 6 | 6 | ||||||||
| Asteraceae | Senecio alloeophyllus O. Hoffm. var. alloeophyllus 1971 | SEAL | EH | NE | 1300–1800 | 31 | 69 | ||||||||||||
| Asteraceae | Senecio humifusus (Hook. f.) Cabrera 1969 | SEHU | EH | NE | 900–1000 | 6 | 6 | 50 | 50 | 19 | |||||||||
| Asteraceae | Senecio neaei DC. 1838 | SENE | S | N | 0–2600 | 38 | 38 | 13 | 25 | 31 | 6 | ||||||||
| Brassicaceae | Noccaea magellanica (Comm. ex Poir.) Holub 1998 | NOMA | EH | N | 0–4000 | 6 | 13 | 50 | 44 | 6 | 31 | ||||||||
| Brassicaceae | Xerodraba lycopodioides (Speg.) Skottsb. 1916 | XELY | CU | NE | 0–1400 | 6 | 6 | ||||||||||||
| Calceolariaceae | Calceolaria polyrrhiza Cav. 1799 | CAPO | EH | N | 500–2000 | 13 | 6 | ||||||||||||
| Calyceraceae | Moschopis trilobata Dusén 1907 | MOTR | EH | NE | 500–1500 | 38 | 13 | ||||||||||||
| Caryophyllaceae | Colobanthus lycopodioides Griseb. 1854 | COLY | CU | N | 0–2300 | 31 | 25 | 13 | 25 | ||||||||||
| Caryophyllaceae | Philippiella patagonica Speg. 1897 | PHPA | CU | N | 0–500 | 6 | 38 | 44 | |||||||||||
| Caryophyllaceae | Stellaria debilis d’Urv. 1825 | STDE | PH | N | 0–3500 | 13 | 25 | 31 | 31 | ||||||||||
| Empetraceae | Empetrum rubrum Vahl ex Willd. 1806 | EMRU | SS | N | 0–2800 | 100 | 100 | 75 | 75 | 63 | 69 | 31 | 31 | ||||||
| Ephedraceae | Ephedra chilensis C. Presl 1845 | EPCH | CU | N | 0–4200 | 19 | 25 | 25 | 25 | ||||||||||
| Ericaceae | Gaultheria pumila (L. f.) D.J. Middleton var. pumila 1990 | GAPU | SS | N | 0–1000 | 94 | 100 | 75 | 75 | 31 | 31 | ||||||||
| Fabaceae | Adesmia aphanantha Speg. 1902 | ADAP | PH | N | 200–1500 | 25 | 31 | ||||||||||||
| Fabaceae | Adesmia villosa Hook. f. 1845 | ADVI | PH | N | 0–2400 | 6 | 31 | 50 | 31 | ||||||||||
| Fabaceae | Astragalus nivicola Gómez-Sosa 1977 | ASNI | PH | N | 1000–2000 | 6 | 38 | ||||||||||||
| Fabaceae | Astragalus palenae (Phil.) Reiche 1897 | ASPA | PH | N | 500–2000 | 31 | 13 | ||||||||||||
| Fabaceae | Vicia magellanica Hook. f. 1846 | VIMA | PH | N | 0–500 | 13 | 19 | 13 | |||||||||||
| Gunneraceae | Gunnera magellanica Lam. 1789 | GUMA | PH | N | 0–1800 | 6 | 6 | ||||||||||||
| Iridaceae | Olsynium biflorum (Thunb.) Goldblatt 1990 | OLBI | EH | NE | 0–1000 | 25 | 25 | 6 | |||||||||||
| Juncaceae | Marsippospermum grandiflorum L. f.) Hook. f. 1843 | MAGR | CG | N | 0–1000 | 19 | 19 | ||||||||||||
| Juncaceae | Luzula alopecurus Desv. 1808 | LUAL | CG | N | 0–1600 | 13 | 25 | 38 | 44 | 31 | 25 | 6 | 6 | ||||||
| Lycopodiaceae | Austrolycopodium magellanicum (P. Beauv.) Holub 1991 | AUMA | F | N | 0–1600 | 31 | 31 | 56 | 56 | ||||||||||
| Montiaceae | Calandrinia caespitosa Gillies ex Arn. 1831 | CACA | EH | N | 300–4000 | 6 | |||||||||||||
| Oxalidaceae | Oxalis enneaphylla Cav.1799 | OXEN | EH | N | 0–2600 | 13 | 13 | 25 | 6 | ||||||||||
| Oxalidaceae | Oxalis loricata Dusén 1901 | OXLO | EH | NE | 0–1500 | 31 | 25 | ||||||||||||
| Poaceae | Bromus catharticus Vahl 1791 | BRCA | CG | N | 0–1000 | 13 | |||||||||||||
| Poaceae | Bromus setifolius J. Presl var. setifolius 1830 | BRSE | CG | N | 0–500 | 69 | 69 | 63 | 38 | 19 | 6 | ||||||||
| Poaceae | Dactylis glomerata L. 1753 | DAGL | CG | E | 0–2000 | 6 | |||||||||||||
| Poaceae | Deschampsia parvula (Hook. f.) E. Desv. 1854 | DEPA | CG | NE | 0–1100 | 38 | 19 | ||||||||||||
| Poaceae | Festuca contracta Kirk 1895 | FECO | CG | N | 200–1100 | 75 | 88 | 56 | 69 | 31 | 31 | 13 | 13 | ||||||
| Poaceae | Festuca pallescens (St.-Yves) Parodi 1953 | FEPA | CG | N | 0–1800 | 25 | 25 | 25 | 31 | ||||||||||
| Poaceae | Hordeum comosum J. Presl 1830 | HOCO | CG | N | 0–4300 | 38 | 31 | 6 | 6 | ||||||||||
| Poaceae | Ortachne rariflora (Hook. f.) Hughes 1923 | ORRA | CG | N | 0–1300 | 56 | 50 | 50 | 38 | ||||||||||
| Poaceae | Pappostipa chrysophylla E. Desv.) Romasch. var. chrysophylla 2008 | PACH | CG | N | 500–4300 | 13 | 50 | 31 | 50 | ||||||||||
| Poaceae | Phleum alpinum L. 1753 | PHAL | CG | N | 0–500 | 6 | |||||||||||||
| Poaceae | Poa alopecurus (Gaudich. ex Mirb.) Kunth subsp. fuegiana (Hook. f.) D.M. Moore & Dogg. 1976 | POAL | CG | N | 0–1300 | 6 | 31 | 38 | 25 | 25 | |||||||||
| Poaceae | Poa pratensis L. 1753 | POPR | RG | E | 0–3800 | 6 | |||||||||||||
| Poaceae | Poa secunda J. Presl 1830 | POSE | CG | NE | 0–600 | 94 | 69 | 81 | 88 | 50 | |||||||||
| Poaceae | Rytidosperma virescens (E. Desv.) Nicora 1973 | RYVI | CG | N | 0–3400 | 6 | |||||||||||||
| Poaceae | Trisetum (L.) K. Richt. subsp. cumingii (Nees ex Steud.) Finot 1890 | TRCU | CG | N | 0–1100 | 13 | 6 | ||||||||||||
| Poaceae | Trisetum spicatum (L.) K. Richt. subsp. spicatum 1890 | TRSP | CG | N | 0–4700 | 6 | 63 | 13 | 38 | ||||||||||
| Ranunculaceae | Caltha dioneifolia Hook. f. 1843 | CADI | CU | NE | 0–1100 | 13 | 13 | ||||||||||||
| Ranunculaceae | Hamadryas delfinii Phil. ex Reiche 1984 | HADE | EH | N | 0–1100 | 13 | 25 | ||||||||||||
| Rosaceae | Acaena antarctica Hook. f. 1846 | ACAN | PH | N | 1000–1600 | 19 | 19 | ||||||||||||
| Rosaceae | Acaena platyacantha Speg. 1897 | ACPL | PH | N | 900–2000 | 38 | 50 | 38 | 44 | ||||||||||
| Rubiaceae | Oreopolus glacialis (Poepp.) Ricardi 1963 | ORGL | CU | N | 300–3500 | 38 | 19 | ||||||||||||
| Schoepfiaceae | Arjona tuberosa Cav. var. tuberosa 1799 | ARTU | EH | N | 0–1500 | 6 | 13 | 63 | 75 | 19 | 19 | ||||||||
| Solanaceae | Benthamiella spegazziniana A. Soriano 1948 | BESP | CU | NE | 1000–1500 | 38 | 44 | ||||||||||||
| Thymelaceae | Drapetes muscosus Lam. 1792 | DRMU | CU | N | 0–1000 | 69 | 81 | 38 | 38 | 25 | 6 | ||||||||
| Valerianaceae | Valeriana moyanoi Speg. 1897 | VAMO | EH | N | 1000–2500 | 19 | 25 | ||||||||||||
| Valerianaceae | Valeriana sedifolia d’Urv. 1825 | VASE | CU | NE | 500–1500 | 6 | |||||||||||||
| Violaceae | Viola tridentata Sm. 1819 | VITR | PH | N | 0–1000 | 38 | 44 | 19 | 19 | ||||||||||
| Unknown | Unknown 01 | UNKN | CU | N | - | 13 | |||||||||||||
Appendix B







References
- Grytnes, J.A.; Kapfer, J.; Jurasinski, G.; Birks, H.H.; Henriksen, H.; Klanderud, K.; Odland, A.; Ohlson, M.; Wipf, S.; Birks, H.J.B. Identifying the driving factors behind observed elevational range shifts on European mountains. Glob. Ecol. Biogeogr. 2014, 23, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Aalto, J.; le Roux, P.C.; Luoto, M. Vegetation Mediates Soil Temperature and Moisture in Arctic-Alpine Environments. Arct. Antarct. Alp. Res. 2013, 45, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Pauli, H.; Gottfried, M.; Lamprecht, A.; Niessner, S.; Rumpf, S.; Winkler, M.; Steinbauer, K.; Grabherr, G. The GLORIA Field Manual—Standard Multi-Summit Approach, Supplementary Methods and Extra Approaches, 5th ed.; GLORIA-Coordination, Austrian Academy of Sciences & University of Natural Resources and Life Sciences: Vienna, Austria, 2015. [Google Scholar]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 2nd ed.; Springer: Heidelberg, Germany, 2003. [Google Scholar]
- Raupach, M.R.; Finnigan, J.J. The influence of topography on meteorological variables and surface-atmosphere interactions. J. Hydrol. 1997, 190, 182–213. [Google Scholar] [CrossRef]
- Löffler, J. Snow cover dynamics, soil moisture variability and vegetation ecology in high mountain catchments of central Norway. Hydrol. Process. 2005, 19, 2385–2405. [Google Scholar] [CrossRef]
- Scherrer, D.; Körner, C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011, 38, 406–416. [Google Scholar] [CrossRef]
- Kazakis, G.; Ghosn, D.; Vogiatzakis, I.N.; Papanastasis, V.P. Vascular plant diversity and climate change in the alpine zone of the Lefka Ori, Crete. Biodiv. Conserv. 2007, 16, 1603–1615. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.R.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Salick, J.; Ghimire, S.K.; Fang, Z.; Dema, S.; Konchar, K.M. Himalayan alpine vegetation, climate change and mitigation. J. Ethnobiol. 2014, 34, 276–293. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.; Lamprecht, A.; Steinbauer, K.; Hulber, K.; Theurillat, J.P.; Breiner, F.; Choler, P.; Ertl, S.; Gutierrez Giron, A.; Rossi, G.; et al. The rich sides of mountain summits—A pan-European view on aspect preferences of alpine plants. J. Biogeogr. 2016, 43, 2261–2273. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Grytnes, J.A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef]
- Cuesta, F.; Muriel, P.; Llambí, L.D.; Halloy, S.; Aguirre, N.; Beck, S.; Carilla, J.; Meneses, R.I.; Cuello, S.; Grau, A.; et al. Latitudinal and altitudinal patterns of plant community diversity on mountain summits across the tropical Andes. Ecography 2017, 40, 1381–1394. [Google Scholar] [CrossRef]
- Cuesta, F.; Tovar, C.; Llambí, L.D.; Gosling, W.D.; Halloy, S.; Carilla, J.; Muriel, P.; Meneses, R.I.; Beck, S.; Ulloa-Ulloa, C.; et al. Thermal niche traits of high alpine plant species and communities across the tropical Andes and their vulnerability to global warming. J. Biogeogr. 2020, 47, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Carilla, J.; Halloy, S.; Cuello, S.; Grau, A.; Malizia, A.; Cuesta, F. Vegetation trends over eleven years on mountain summits in NW Argentina. Ecol. Evol. 2018, 8, 11554–11567. [Google Scholar] [CrossRef] [PubMed]
- Fadrique, B.; Báez, S.; Duque, A.; Malizia, A.; Blundo, C.; Carilla, J.; Osinaga-Acosta, O.; Malizia, L.; Silman, M.; Farfán-Ríos, W.; et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature 2018, 564, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Venn, S.; Pickering, C.; Green, K. Short-term variation in species richness across an altitudinal gradient of alpine summits. Biodiv. Conserv. 2012, 21, 3157–3186. [Google Scholar] [CrossRef]
- Philander, S.G.H. El Niño Southern Oscillation phenomena. Nature 1983, 302, 295–301. [Google Scholar] [CrossRef]
- Rintoul, S.R.; Hughes, C.; Olbers, D. The Antarctic Circumpolar Current System. In Ocean Circulation and Climate; Siedler, G., Church, J., Gould, J., Eds.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Mohtadi, M.; Romero, O.E.; Kaiser, J.; Hebbeln, D. Cooling of the southern high latitudes during the Medieval Period and its effect on ENSO. Quat. Sci. Rev. 2007, 26, 1055–1066. [Google Scholar] [CrossRef]
- Lencinas, M.V.; Martínez Pastur, G.; Rivero, P.; Busso, C.A. Conservation value of timber quality versus associated non-timber quality stands for understory diversity in Nothofagus forests. Biod. Conserv. 2008, 17, 2579–2597. [Google Scholar] [CrossRef]
- Peri, P.L.; Lencinas, M.V.; Martínez Pastur, G.; Wardell-Johnson, G.W.; Lasagno, R. Diversity patterns in the steppe of Argentinean Southern Patagonia: Environmental drivers and impact of grazing. In Steppe Ecosystems: Biological Diversity, Management and Restoration; Morales Prieto, M.B., Traba Díaz, J., Eds.; NOVA Science Publishers Inc.: New York, NY, USA, 2013; pp. 73–95. [Google Scholar]
- Golluscio, R.A.; Deregibus, V.; Paruelo, J.M. Sustainability and range management in the Patagonian steppe. Ecol. Aust. 1998, 8, 265–284. [Google Scholar]
- Martínez Pastur, G.; Peri, P.L.; Lencinas, M.V.; García-Llorente, M.; Martín-López, B. Spatial patterns of cultural ecosystem services provision in Southern Patagonia. Landsc. Ecol. 2016, 31, 383–399. [Google Scholar] [CrossRef]
- Correa, M.N. Flora Patagónica. In Colección Científica INTA. Partes II, III, IVb, V, VI y VII; Ediciones INTA: Buenos Aires, Argentina; pp. 1969–1998.
- Moore, D.M. The alpine flora of Tierra del Fuego. An. Inst. Bot. Cavanilles 1975, 32, 419–440. [Google Scholar]
- Moore, D.M. Flora of Tierra del Fuego; Anthony Nelson: Oswestry, Shropshire, UK; Missouri Botanical Garden: St. Louis, MO, USA, 1983. [Google Scholar]
- Boelcke, O.; Moore, D.M.; Roig, F.A. Transecta Botánica de la Patagonia Austral; CONICET: Buenos Aires, Argentina; Royal Society: London, UK; Instituto de la Patagonia: Punta Arenas, Chile, 1985. [Google Scholar]
- León, R.J.; Bran, D.; Collantes, M.; Paruelo, J.M.; Soriano, A. Grandes unidades de vegetación de la Patagonia extra andina. Ecol. Aust. 1998, 8, 125–144. [Google Scholar]
- Mark, A.F.; Dickinson, K.J.M.; Allen, J.; Smith, R.; West, C.J. Vegetation patterns, plant distribution and life forms across the alpine zone in southern Tierra del Fuego, Argentina. Aust. Ecol. 2001, 26, 423–440. [Google Scholar] [CrossRef]
- Brancaleoni, L.; Strelin, J.; Gerdol, R. Relationships between geomorphology and vegetation patterns in subantarctic Andean tundra of Tierra del Fuego. Polar Biol. 2003, 26, 404–410. [Google Scholar] [CrossRef]
- Peri, P.L.; Lencinas, M.V.; Bousson, J.; Lasagno, R.; Soler, R.; Bahamonde, H.; Martínez Pastur, G. Biodiversity and ecological long-term plots in Southern Patagonia to support sustainable land management: The case of PEBANPA network. J. Nat. Conserv. 2016, 34, 51–64. [Google Scholar] [CrossRef]
- Oliva, G.; González, L.; Rial, P.; Livraghi, E. El Ambiente en la Patagonia Austral. Ganadería Ovina Sustentable en la Patagonia Austral. 2001, pp. 17–80. Available online: https://www.researchgate.net/profile/Gabriel-Oliva/publication/313631051_Areas_ecologicas_de_Santa_Cruz_y_Tierra_del_Fuego/links/594a7f82458515225a82f296/Areas-ecologicas-de-Santa-Cruz-y-Tierra-del-Fuego.pdf (accessed on 30 May 2021).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Kreps, G.; Martínez Pastur, G.; Peri, P. Cambio Climático en Patagonia sur. Escenarios Futuros en el Manejo de los Recursos Naturales; Ediciones INTA: Buenos Aires, Argentina, 2012. [Google Scholar]
- Gea, G.; Martínez Pastur, G.; Cellini, J.M.; Lencinas, M.V. Forty years of silvicultural management in southern Nothofagus pumilio (Poepp. et Endl.) Krasser primary forests. For. Ecol. Manag. 2004, 201, 335–347. [Google Scholar]
- Zuloaga, F.O.; Belgrano, M.J.; Zanotti, C.A. Actualización del Catálogo de las Plantas Vasculares del Cono Sur. Darwiniana Nueva Ser. 2019, 7, 208–278. [Google Scholar] [CrossRef]
- Wolter, K.; Timlin, M.S. Measuring the strength of ENSO events: How does 1997/98 rank? Weather 1998, 53, 315–324. [Google Scholar] [CrossRef]
- Pielou, E.C. Mathematical Ecology; John Wiley and Sons Inc.: New York, NY, USA, 1975. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Zimmerman, G.M.; Goetz, H.; Mielke, P.W. Use of an improved statistical-method for group comparisons to study effects of prairie fire. Ecology 1985, 66, 606–611. [Google Scholar] [CrossRef]
- Noroozi, J.; Körner, C. A bioclimatic characterization of high elevation habitats in the Alborz mountains of Iran. Alp. Bot. 2018, 128, 1–11. [Google Scholar] [CrossRef] [Green Version]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data. Version 5.0; MjM Software: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Sklenář, P.; Ramsay, P.M. Diversity of zonal páramo plant communities in Ecuador. Div. Distr. 2001, 7, 113–124. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P.B. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of South-Central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- McDougall, K.L.; Morgan, J.H.; Walsh, N.G.; Williams, R.J. Plant invasions in treeless vegetation of the Australian Alps. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 159–171. [Google Scholar] [CrossRef]
- Steinmann, V.W.; Arredondo-Amezcua, L.; Hernández-Cárdenas, R.A.; Ramírez-Amezcua, Y. Diversity and origin of the Central Mexican alpine flora. Diversity 2021, 13, 31. [Google Scholar] [CrossRef]
- Speed, J.D.M.; Austrheim, G.; Hester, A.J.; Mysterud, A. Elevational advance of alpine plant communities is buffered by herbivory. J. Veg. Sci. 2012, 23, 617–625. [Google Scholar] [CrossRef]
- Vásquez, D.L.A.; Balslev, H.; Sklenář, P. Human impact on tropical-alpine plant diversity in the northern Andes. Biod. Conserv. 2015, 24, 2673–2683. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate effects on mountain plants. Nature 1994, 369, 448. [Google Scholar] [CrossRef] [PubMed]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob. Chang. Biol. 2007, 13, 146–156. [Google Scholar] [CrossRef]
- Kazakis, G.; Ghosn, D.; Remoundou, I.; Nyktas, P.; Talias, M.A.; Vogiatzakis, I.N. Altitudinal vascular plant richness and climate change in the alpine zone of the Lefka Ori, Crete. Diversity 2021, 13, 22. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.-C.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.E.; Dullinger, S.; Pauli, H.; Willner, W.; Svenning, J.-C. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 2010, 33, 295–303. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Klonner, G.; Moser, D.; Schütz, M.; Wessely, J.; Willner, W.; Zimmermann, N.E.; Dullinger, S. Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. USA 2018, 115, 1848–1853. [Google Scholar] [CrossRef] [Green Version]
- Essl, F.; Dullinger, S.; Rabitsch, W.; Hulme, P.E.; Pyšek, P.; Wilson, J.R.U.; Richardson, D.M. Delayed Biodiversity Change: No Time to Waste. Trends Ecol. Evol. 2015, 30, 375–378. [Google Scholar] [CrossRef]
- Davis, E.L.; Brown, R.; Daniels, L.; Kavanagh, T.; Gedalof, Z. Regional variability in the response of alpine treelines to climate change. Clim. Chang. 2020, 162, 1365–1384. [Google Scholar] [CrossRef]
- Larcher, W.; Kainmüller, C.; Wagner, J. Survival types of high mountain plants under extreme temperatures. Flora 2010, 205, 3–18. [Google Scholar] [CrossRef]
- Neuner, G.; Huber, B.; Plangger, A.; Pohlin, J.M.; Walde, J. Low temperatures at higher elevations require plants to exhibit increased freezing resistance throughout the summer months. Env. Exp. Bot. 2020, 169, 103882. [Google Scholar] [CrossRef]
- Billings, W.D. Adaptations and origins of alpine plants. Arct. Alp. Res. 1974, 6, 129–142. [Google Scholar] [CrossRef]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel competitors shape species’ responses to climate change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Tovar, C.; Melcher, I.; Kusumoto, B.; Cuesta, F.; Cleef, A.; Meneses, R.I.; Halloy, S.; Llambí, L.D.; Beck, S.; Muriel, P.; et al. Plant dispersal strategies of high tropical alpine communities across the Andes. J. Ecol. 2020, 108, 1910–1922. [Google Scholar] [CrossRef]
- Jonas, T.; Rixen, C.; Sturm, M.; Stoeckli, V. How alpine plant growth is linked to snow cover and climate variability. J. Geophys. Res. 2008, 113, G03013. [Google Scholar] [CrossRef] [Green Version]
- Choler, P. Winter soil temperature dependence of alpine plant distribution: Implications for anticipating vegetation changes under a warming climate. Per. Plant Ecol. Evol. Syst. 2018, 30, 6–15. [Google Scholar] [CrossRef]
- Trask, J.C.; Devine, S.M.; Fogg, G.E. Soil temperature survey in a mountain basin. Geoderma 2020, 367, 114202. [Google Scholar] [CrossRef]
- Miehe, G. Vegetation patterns on Mount Everest as influenced by monsoon and föhn. Vegetatio 1989, 79, 21–32. [Google Scholar] [CrossRef]
- Borrelli, P.; Oliva, G. Efectos de los Animales Sobre los Pastizales. Ganadería Ovina Sustentable en la Patagonia Austral. 2001, pp. 99–128. Available online: https://www.researchgate.net/profile/Gabriel-Oliva/publication/242658835_Efectos_de_los_animales_sobre_los_pastizales/links/00b4952c5fc16b9b44000000/Efectos-de-los-animales-sobre-los-pastizales.pdf (accessed on 30 May 2021).
- Roig, F.; Anchorena, J.; Dollenz, A.; Faggi, A.; Méndez, E. Las comunidades vegetales de la transecta botánica de la Patagonia Austral. Primera parte. La vegetación del área continental. In Transecta Botánica de la Patagonia Austral; Boelcke, O., Moore, D.M., Roig, F., Eds.; CONICET: Buenos Aires, Argentina; Royal Society: London, UK; Instituto de la Patagonia: Punta Arenas, Chile, 1985. [Google Scholar]
- Crimmins, S.M.; Dobrowski, S.Z.; Greenberg, J.A.; Abatzoglou, J.T.; Mynsberge, A.R. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 2011, 331, 324–327. [Google Scholar] [CrossRef] [PubMed]




| Family | Santa Cruz | Tierra del Fuego | |||||
|---|---|---|---|---|---|---|---|
| L | M | H | L | ML | MH | H | |
| Alstroemeriaceae | <0.01 (1) | <0.01 (1) | |||||
| Amaryllidaceae | 0.03 (1) | ||||||
| Apiaceae | 0.3 (1) | 0.3 (1) | 1.7 (2) | 12.2 (4) | 8.8 (3) | 3.7 (3) | 0.2 (1) |
| Asteraceae | 18.7 (7) | 12.3 (6) | 2.6 (11) | 1.3 (3) | 2.8 (4) | 0.8 (5) | 1.5 (2) |
| Brassicaceae | <0.01 (1) | 0.1 (1) | 0.02 (2) | ||||
| Calceolariaceae | 0.01 (1) | ||||||
| Calyceraceae | <0.01 (1) | ||||||
| Caryophyllaceae | 1.0 (3) | 0.3 (3) | |||||
| Empetraceae | 64.3 (1) | 45.9 (1) | 20.0 (1) | 6.6 (1) | |||
| Ephedraceae | 0.02 (1) | 0.5 (1) | |||||
| Ericaceae | 3.2 (1) | 3.3 (1) | 1.4 (1) | ||||
| Fabaceae | 0.08 (3) | 0.6 (3) | 0.3 (2) | ||||
| Gunneraceae | 0.09 (1) | ||||||
| Iridaceae | <0.01 (1) | <0.01 (1) | |||||
| Juncaceae | 1.0 (2) | 0.1 (1) | 0.09 (1) | 0.09 (1) | |||
| Lycopodiaceae | 0.4 (1) | 1.1 (1) | |||||
| Montiaceae | 0.01 (1) | ||||||
| Oxalidaceae | 0.2 (1) | 0.1 (2) | |||||
| Poaceae | 10.2 (9) | 18.9 (5) | 0.6 (3) | 11.3 (5) | 5.1 (5) | 0.9 (4) | 0.2 (1) |
| Ranunculaceae | 0.07 (1) | 0.08 (1) | |||||
| Rosaceae | 0.7 (1) | 0.4 (1) | 1.0 (1) | ||||
| Rubiaceae | 0.2 (1) | ||||||
| Schoepfiaceae | 0.01 (1) | 0.1 (1) | 0.03 (1) | ||||
| Solanaceae | 0.9 (1) | ||||||
| Thymelaceae | 1.0 (1) | 0.7 (1) | 0.2 (1) | ||||
| Valerianaceae | <0.01 (1) | 0.4 (2) | |||||
| Violaceae | 0.3 (1) | 0.08 (1) | |||||
| Unknown family | <0.01 (1) | ||||||
| Total | 31.2 (31) | 34.3 (26) | 6.0 (30) | 95.0 (21) | 67.8 (18) | 28.0 (18) | 8.6 (6) |
| Site | Factor | Level | Richness (n° sp.) | Cover (%) | xxxShannon-Wiener (SW) | Pielou (J) |
|---|---|---|---|---|---|---|
| Santa Cruz | A: Elevation | L | 7.4 b | 31.2 b | 1.11 b | 0.56 b |
| M | 9.0 c | 34.3 b | 1.10 b | 0.50 ab | ||
| H | 5.6 a | 6.0 a | 0.84 a | 0.44 a | ||
| F (p) | 36.59 (<0.01) | 123.83 (<0.01) | 8.33 (<0.01) | 3.31 (0.04) | ||
| B: Aspect | North | 4.4 a | 16.1 a | 0.62 a | 0.39 a | |
| East | 8.8 c | 33.6 c | 1.17 c | 0.53 bc | ||
| South | 9.1 c | 19.1 a | 1.37 d | 0.63 c | ||
| West | 7.2 b | 26.6 b | 0.93 b | 0.45 ab | ||
| F (p) | 45.16 (<0.01) | 23.70 (<0.01) | 31.18 (<0.01) | 8.58 (<0.01) | ||
| C: Sampling date | BL | 7.0 a | 22.3 | 0.94 a | 0.44 a | |
| RS | 7.7 b | 25.4 | 1.10 b | 0.55 b | ||
| F (p) | 4.28 (0.04) | 3.8 (0.06) | 8.15 (0.01) | 9.62 (<0.01) | ||
| A × B | F (p) | 50.48 (<0.01) | 22.70 (<0.01) | 34.61 (<0.01) | 11.56 (<0.01) | |
| A × C | F (p) | 0.1 (0.90) | 0.17 (0.84) | 0.7 (0.50) | 3.82 (0.03) | |
| B × C | F (p) | 1.73 (0.17) | 2.59 (0.06) | 1.29 (0.28) | 0.05 (0.98) | |
| A × B × C | F (p) | 1.58 (0.17) | 3.26 (<0.01) | 0.59 (0.74) | 1.94 (0.09) | |
| Tierra del Fuego | A: Elevation | L | 8.6 d | 94.6 d | 0.82 c | 0.39 ab |
| ML | 7.3 c | 67.8 c | 0.82 c | 0.44 b | ||
| MH | 3.9 b | 28.0 b | 0.54 b | 0.33 ab | ||
| H | 1.1 a | 8.6 a | 0.21 a | 0.25 a | ||
| F (p) | 101.64 (<0.01) | 135.83 (<0.01) | 14.26 (<0.01) | 2.9 (0.04) | ||
| B: Aspect | North | 5.8 b | 50.0 ab | 0.66 ab | 0.34 | |
| East | 4.0 a | 64.7 c | 0.39 a | 0.30 | ||
| South | 5.6 b | 50.1 b | 0.66 ab | 0.40 | ||
| West | 5.6 b | 37.7 a | 0.67 b | 0.37 | ||
| F (p) | 6.25 (<0.01) | 11.56 (<0.01) | 3.61 (0.02) | 1.02 (0.40) | ||
| C: Sampling date | BL | 4.9 a | 50.3 | 0.65 | 0.40 b | |
| RS | 5.6 b | 49.3 | 0.54 | 0.30 a | ||
| F (p) | 4.85 (0.03) | 0.09 (0.77) | 2.26 (0.14) | 5.21 (0.03) | ||
| A × B | F (p) | 12.29 (<0.01) | 5.18 (<0.01) | 5.72 (<0.01) | 5.91 (<0.01) | |
| A × C | F (p) | 1.06 (0.37) | 0.35 (0.79) | 0.19 (0.91) | 0.18 (0.91) | |
| B × C | F (p) | 0.42 (0.74) | 0.26 (0.86) | 0.84 (0.48) | 0.87 (0.46) | |
| A × B × C | F (p) | 0.6 (0.79) | 0.31 (0.97) | 0.55 (0.83) | 0.85 (0.56) |
| Site | Factor | Group Comparison | MRPP Statistics | ||
|---|---|---|---|---|---|
| T | A | p | |||
| Santa Cruz | Elevation | Overall | −5.63 | 0.16 | <0.01 |
| L vs. M | −1.68 | 0.05 | 0.07 | ||
| L vs. H | −4.59 | 0.19 | <0.01 | ||
| M vs. H | −4.56 | 0.18 | <0.01 | ||
| Aspect | Overall | −4.61 | 0.17 | <0.01 | |
| East vs. North | −3.61 | 0.19 | <0.01 | ||
| East vs. South | −3.13 | 0.11 | <0.01 | ||
| East vs. West | −4.18 | 0.21 | <0.01 | ||
| North vs. South | −0.97 | 0.05 | 0.16 | ||
| North vs. West | −0.14 | 0.01 | 0.34 | ||
| South vs. West | −0.12 | 0.05 | 0.12 | ||
| Sampling date | Overall = BL vs. RS | 1.64 | −0.03 | 1.00 | |
| Tierra del Fuego | Elevation | Overall | −7.95 | 0.21 | <0.01 |
| L vs. ML | −0.88 | 0.03 | 0.18 | ||
| L vs. MH | −4.80 | 0.17 | <0.01 | ||
| L vs. H | −8.27 | 0.29 | <0.01 | ||
| ML vs. MH | −2.57 | 0.09 | 0.03 | ||
| ML vs. H | −6.42 | 0.21 | <0.01 | ||
| MH vs. H | −2.59 | 0.07 | 0.02 | ||
| Aspect | Overall | −2.53 | 0.07 | 0.02 | |
| East vs. North | −0.83 | 0.03 | 0.17 | ||
| East vs. South | −0.59 | 0.03 | 0.21 | ||
| East vs. West | −5.11 | 0.15 | <0.01 | ||
| North vs. South | 0.36 | −0.01 | 0.55 | ||
| North vs. West | −1.50 | 0.04 | 0.08 | ||
| South vs. West | −1.07 | 0.03 | 0.14 | ||
| Sampling date | Overall = BL vs. RS | 1.57 | −0.02 | 1.00 | |
| Site | Factor | Group | Species | IndVal | p |
|---|---|---|---|---|---|
| Santa Cruz | Elevation | L | Bromus setifolius var. setifolius | 64.8 | 0.022 |
| Nassauvia glomerulosa | 58.6 | 0.015 | |||
| M | Benthamiella spegazziniana | 75.0 | <0.001 | ||
| Nardophyllum bryoides | 59.3 | 0.046 | |||
| Arjona tuberosa var. tuberosa | 57.9 | 0.019 | |||
| Philippiella patagonica | 45.3 | 0.036 | |||
| H | Oxalis loricata | 50.0 | 0.019 | ||
| Adesmia aphanantha | 50.0 | 0.021 | |||
| Astragalus nivicola | 50.0 | 0.021 | |||
| Asteraceae 03 | 50.0 | 0.021 | |||
| Nassauvia darwinii | 50.0 | 0.021 | |||
| Perezia pilifera | 50.0 | 0.021 | |||
| Tristagma nivale | 50.0 | 0.021 | |||
| Aspect | E | Senecio neaei | 81.8 | <0.001 | |
| Nassauvia aculeata var. azorelloides | 79.0 | 0.002 | |||
| Azorella monantha | 79.0 | 0.001 | |||
| Festuca pallescens | 66.5 | 0.005 | |||
| Hordeum comosum | 56.5 | 0.21 | |||
| Vicia magellanica | 50.0 | 0.038 | |||
| N | - | - | - | ||
| W | Colobanthus lycopodioides | 64.8 | 0.015 | ||
| Perezia recurvata | 49.7 | 0.039 | |||
| S | Oxalis enneaphylla | 66.7 | 0.007 | ||
| Noccaea magellanica | 60.6 | 0.033 | |||
| Sampling date | BL | - | - | - | |
| RS | - | - | - | ||
| Tierra del Fuego | Elevation | L | Ortachne rariflora | 71.1 | 0.004 |
| Bolax gummifera | 61.9 | 0.002 | |||
| Drapetes muscosus | 51.5 | 0.007 | |||
| Empetrum rubrum | 47.0 | 0.002 | |||
| ML | Abrotanella emarginata | 68.9 | <0.001 | ||
| Austrolycopodium magellanicum | 54.6 | 0.002 | |||
| MH | Senecio humifusus | 52.9 | 0.009 | ||
| H | Senecio alloeophyllus var. alloeophyllus | 87.5 | <0.001 | ||
| Aspect | E | Empetrum rubrum | 40.6 | 0.048 | |
| N | - | - | - | ||
| S | - | - | - | ||
| W | - | - | - | ||
| Sampling date | BL | - | - | - | |
| RS | Deschampsia parvula | 37.5 | 0.017 |
| Site | Factor | Level | Tmin (°C) | Tmean (°C) | Tmax (°C) |
|---|---|---|---|---|---|
| Santa Cruz | A: Elevation | L | 0.2 b | 6.8 b | 16.5 b |
| M | −0.1 b | 5.8 ab | 14.7 ab | ||
| H | −0.9 a | 4.9 a | 13.8 a | ||
| F (p) | 7.30 (<0.01) | 7.93 (<0.01) | 6.53 (<0.01) | ||
| B: Aspect | North | 0.1 | 6.4 | 16.4 | |
| East | 0.0 | 6.0 | 14.6 | ||
| South | −0.6 | 5.4 | 14.2 | ||
| West | −0.5 | 5.6 | 14.9 | ||
| F (p) | 2.34 (0.07) | 1.34 (0.26) | 2.26 (0.08) | ||
| C: Annual period | 2014–2015 | 0.3 | 5.9 | 13.6 a | |
| 2015–2016 | −0.1 | 6.1 | 15.4 ab | ||
| 2016–2017 | −0.8 | 6.1 | 17.1 b | ||
| 2017–2018 | −0.6 | 5.4 | 14.9 ab | ||
| 2018–2019 | −0.1 | 5.7 | 14.1 a | ||
| F (p) | 2.35 (0.05) | 0.48 (0.75) | 3.69 (<0.01) | ||
| A × B | F (p) | 1.90 (0.08) | 0.18 (0.98) | 0.87 (0.51) | |
| A × C | F (p) | 1.49 (0.16) | 0.02 (>0.99) | 0.76 (0.64) | |
| B × C | F (p) | 0.66 (0.79) | 0.02 (>0.99) | 0.21 (0.99) | |
| A × B × C | F (p) | 0.31 (0.99) | 0.01 (>0.99) | 0.16 (>0.99) | |
| Tierra del Fuego | A: Elevation | L | 1.0 c | 2.8 c | 6.0 ab |
| ML | 0.8 c | 2.5 bc | 5.8 a | ||
| MH | −1.2 b | 1.9 ab | 7.8 c | ||
| H | −2.3 a | 1.3 a | 7.3 bc | ||
| F (p) | 30.22 (<0.01) | 11.89 (<0.01) | 6.15 (<0.01) | ||
| B: Aspect | North | −0.2 | 2.3 | 7.3 b | |
| East | −0.5 | 2.2 | 6.7 ab | ||
| South | −0.5 | 1.7 | 5.5 a | ||
| West | −0.6 | 2.2 | 7.5 b | ||
| F (p) | 2.32 (0.07) | 1.78 (0.15) | 5.13 (<0.01) | ||
| C: Annual period | 2013–2014 | −0.8 a | 1.6 a | 6.2 | |
| 2014–2015 | −0.6 ab | 2.1 ab | 6.9 | ||
| 2015–2016 | −0.8 a | 1.9 a | 6.6 | ||
| 2016–2017 | 0.2 c | 2.8 b | 7.4 | ||
| 2017–2018 | −0.3 b | 2.3 ab | 6.6 | ||
| F (p) | 12.05 (<0.01) | 4.05 (<0.01) | 0.94 (0.44) | ||
| A × B | F (p) | 8.72 (<0.01) | 0.42 (0.93) | 2.07 (0.03) | |
| A × C | F (p) | 0.63 (0.81) | 0.02 (>0.99) | 0.23 (0.99) | |
| B × C | F (p) | 0.19 (0.99) | 0.20 (0.91) | 0.08 (>0.99) | |
| A × B × C | F (p) | 0.25 (0.99) | 0.01 (>0.99) | 0.08 (>0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lencinas, M.V.; Soler, R.; Cellini, J.M.; Bahamonde, H.; Pérez Flores, M.; Monelos, L.; Martínez Pastur, G.J.; Peri, P.L. Variation in Alpine Plant Diversity and Soil Temperatures in Two Mountain Landscapes of South Patagonia. Diversity 2021, 13, 310. https://doi.org/10.3390/d13070310
Lencinas MV, Soler R, Cellini JM, Bahamonde H, Pérez Flores M, Monelos L, Martínez Pastur GJ, Peri PL. Variation in Alpine Plant Diversity and Soil Temperatures in Two Mountain Landscapes of South Patagonia. Diversity. 2021; 13(7):310. https://doi.org/10.3390/d13070310
Chicago/Turabian StyleLencinas, María Vanessa, Rosina Soler, Juan Manuel Cellini, Héctor Bahamonde, Magalí Pérez Flores, Lucas Monelos, Guillermo José Martínez Pastur, and Pablo Luis Peri. 2021. "Variation in Alpine Plant Diversity and Soil Temperatures in Two Mountain Landscapes of South Patagonia" Diversity 13, no. 7: 310. https://doi.org/10.3390/d13070310
APA StyleLencinas, M. V., Soler, R., Cellini, J. M., Bahamonde, H., Pérez Flores, M., Monelos, L., Martínez Pastur, G. J., & Peri, P. L. (2021). Variation in Alpine Plant Diversity and Soil Temperatures in Two Mountain Landscapes of South Patagonia. Diversity, 13(7), 310. https://doi.org/10.3390/d13070310