Socioenvironmental and Spatial Criteria as Tools for the Management and Conservation of Hydrozoans in Protected and Unprotected Areas
Abstract
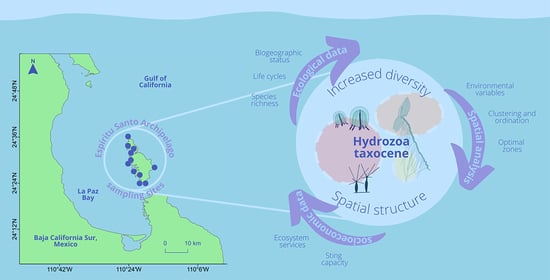
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Data Analysis
2.3. Spatial Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groves, C.R.; Jensen, D.B.; Valutis, L.L.; Redford, K.H.; Shaffer, M.L.; Scott, J.M.; Baumgartner, J.V.; Higgins, J.V.; Beck, M.W.; Anderson, M.G. Planning for Biodiversity Conservation: Putting Conservation Science into Practice: A seven-step framework for developing regional plans to conserve biological diversity, based upon principles of conservation biology and ecology, is being used extensively by the nature conservancy to identify priority areas for conservation. BioScience 2002, 52, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, S.K.; Andersen, J.H.; Berg, T.; Blanchet, H.; Borja, A.; Carstensen, J.; Elliott, M.; Hummel, H.; Niquil, N.; Renaud, P.E. What is marine biodiversity? Towards common concepts and their implications for assessing biodiversity status. Front. Mar. Sci. 2016, 3, 248. [Google Scholar] [CrossRef] [Green Version]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef]
- Zacharias, M.A.; Roff, J.C. A Hierarchical Ecological Approach to Conserving Marine Biodiversity. Conserv. Biol. 2000, 14, 1327–1334. [Google Scholar] [CrossRef]
- Fortin, M.J.; Dale, M. Spatial Analysis: A Guide to Ecologists, 1st ed.; Cambridge University Press: Cambridge, UK, 2005; pp. 1–365. [Google Scholar]
- World Register of Marine Species. Available online: https://www.marinespecies.org/index.php (accessed on 4 December 2022).
- Marques, A.C.; Collins, A.G. Cladistic analysis of Medusozoa and cnidarian evolution. Invertebr. Biol. 2004, 123, 23–42. [Google Scholar] [CrossRef]
- Gibbons, M.J.; Janson, L.A.; Ismail, A.; Samai, T. Life cycle strategy, species richness and distribution in marine Hydrozoa (Cnidaria: Medusozoa). J. Biogeogr. 2010, 37, 441–448. [Google Scholar] [CrossRef]
- Maronna, M.M.; Miranda, T.P.; Peña Cantero, Á.L.; Barbeitos, M.S.; Marques, A.C. Towards a phylogenetic classification of Leptothecata (Cnidaria, Hydrozoa). Sci. Rep. 2016, 6, 18075. [Google Scholar] [CrossRef] [Green Version]
- Kayal, E.; Bentlage, B.; Pankey, M.S.; Ohdera, A.H.; Medina, M.; Plachetzki, D.C.; Collins, A.G.; Ryan, J.F. Phylogenomics provides a robust topology of the major cnidarian lineages and insights. BMC Evol. Biol. 2018, 18, 68. [Google Scholar] [CrossRef] [Green Version]
- Chalfie, M. Green Fluorescent Protein. Photochem. Photobiol. 1995, 62, 651–656. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a Source of New Marine Bioactive Compounds—An Overview of the Last Decade and Future Steps for Bioprospecting. Marine Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Lewis Ames, C. Medusa: A Review of an Ancient Cnidarian Body Form. In Marine Organisms as Model Systems in Biology and Medicine, 1st ed.; Kloc, M., Kubiak, J.Z., Eds.; Springer: Edinburgh, UK, 2018; Volume 65, pp. 106–136. [Google Scholar] [CrossRef]
- Guevara, B.E.K.; Dayrit, J.F.; Haddad, V. Delayed allergic dermatitis presenting as a keloid-like reaction caused by sting from an Indo-Pacific Portuguese man-o’-war (Physalia utriculus). Clin. Exp. Dermatol. 2017, 42, 182–184. [Google Scholar] [CrossRef]
- Santhanam, R. Biology and Ecology of Venomous Marine Cnidarians; Springer: Singapore, 2020; pp. 1–343. [Google Scholar] [CrossRef]
- Doyle, T.K.; Hays, G.C.; Harrod, C.; Houghton, J.D.R. Ecological and Societal Benefits of Jellyfish. In Jellyfish Blooms, 1st ed.; Pitt, K., Lucas, C., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 105–127. [Google Scholar] [CrossRef]
- Coma, R.; Gili, J.; Zabala, M. Trophic ecology of a benthic marine hydroid, Campanularia everta. Mar. Ecol. Prog. Ser. 1995, 119, 211–220. [Google Scholar] [CrossRef]
- Gili, J.; Hughes, R.G. The Ecology of Marine Benthic Hydroids. Oceanogr. Mar. Biol. 1999, 33, 351–426. [Google Scholar]
- Oliveira, O.M.P.; Marques, A.C. Epiphytic hydroids (Hydrozoa: Anthoathecata and Leptothecata) of the World. Check List 2007, 3, 21–38. [Google Scholar] [CrossRef]
- Di Camillo, C.G.; Bavestrello, G.; Cerrano, C.; Gravili, C.; Piraino, S.; Puce, S.; Boero, F. Hydroids (Cnidaria, Hydrozoa): A Neglected Component of Animal Forests. In Marine Animal Forests, 1st ed.; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Edinburgh, UK, 2017; pp. 397–427. [Google Scholar] [CrossRef]
- Okamura, B.; Gruhl, A. Evolution, Origins and Diversification of Parasitic Cnidarians. In The Evolution and Fossil Record of Parasitism. Topics in Geobiology, 1st ed.; De Baets, K., Huntley, J.W., Eds.; Springer: Edinburgh, UK, 2020; Volume 49, pp. 109–152. [Google Scholar] [CrossRef]
- Calder, D.R. Hydroids from Rocas Alijos. In Rocas Alijos Monographiae Biologicae, 1st ed.; Schmieder, R.W., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 257–261. [Google Scholar] [CrossRef]
- Calder, D.R. Additions to the hydroids (Cnidaria, Hydrozoa) of marine fouling communities on the mainland of Ecuador and in the Galapagos Islands. Aquat. Invasions 2021, 16, 208–252. [Google Scholar] [CrossRef]
- Bardi, J.; Marques, A.C. The invasive Hydromedusae Blackfordia virginica Mayer, 1910 (Cnidaria: Blackfordiidae) in southern Brazil, with comments on taxonomy and distribution of the genus Blackfordia. Zootaxa 2009, 2198, 41–50. [Google Scholar] [CrossRef]
- SEMARNAT; CONANP. Programa de Manejo Parque Nacional Revillagigedo, 1st ed.; SEMARNAT; CONANP: Mexico City, México, 2019; pp. 1–342. [Google Scholar]
- SEMARNAT; CONANP. Programa de Conservación y Manejo Parque Nacional Islas Marietas, 1st ed.; SEMARNAT; CONANP: Mexico City, México, 2007; pp. 1–155. [Google Scholar]
- SEMARNAT; CONANP. Programa de Manejo Parque Nacional Exclusivamente la Zona Marina del Archipiélago de Espíritu Santo, 1st ed.; SEMARNAT; CONANP: Mexico City, México, 2014; pp. 1–226. [Google Scholar]
- Decreto por el que se Declara Área Natural Protegida, con la Categoría de Parque Nacional Exclusivamente la Zona Marina del Archipiélago de Espíritu Santo, Ubicado en el Golfo de California, Frente a las Costas del Municipio de La Paz, Baja California Sur. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4987303&fecha=10/05/2007#gsc.tab=0 (accessed on 4 December 2022).
- Enríquez-Andrade, R.; Anaya-Reyna, G.; Barrera-Guevara, J.C.; de los Ángeles Carvajal-Moreno, M.; Martínez-Delgado, M.E.; Vaca-Rodríguez, J.; Valdés-Casillas, C. An analysis of critical areas for biodiversity conservation in the Gulf of California region. Ocean Coast. Manag. 2005, 48, 31–50. [Google Scholar] [CrossRef]
- CONANP. Lineamientos y Directrices para el Desarrollo de Actividades de Monitoreo en las Áreas Naturales Protegidas; SEMARNAT; CONANP: Mexico City, Mexico, 2020; pp. 1–145. [Google Scholar]
- Fraser, C.M. Hydroids of the 1936 and 1937 Allan Hancock Pacific Expeditions. Allan Hancock Exped. 1938, 4, 107–127. [Google Scholar]
- Fraser, C.M. Hydroids of the Allan Hancock Pacific Expeditions since March. Allan Hancock Pac. Exped. 1948, 4, 179–343. [Google Scholar]
- Cairns, S.D.; Barnard, J.L. Redescription of Janaria mirabilis, a Calcified Hydroid from the Eastern Pacific. Bull. South Calif. Acad. Sci. 1984, 83, 1–11. [Google Scholar]
- INEGI. Catálogo de Territorio Insular Mexicano, 1st ed.; Instituto Nacional de Estadística y Geografía: Aguascalientes, Mexico, 2015; pp. 1–243. [Google Scholar]
- González-Medina, F.J.; Holguín-Quiñones, O.E.; de la Cruz-Agüero, G. Variación espaciotemporal de algunos macroinvertebrados (Gastropoda, Bivalvia y Echinodermata) de fondos someros del Archipiélago Espíritu Santo, Baja California Sur, México. Cienc. Mar. 2006, 32, 33–44. [Google Scholar] [CrossRef]
- Ramos García, J.G.; Petatan Ramirez, D.; Reyes Bonilla, H.; Luna Soria, H.; Gonzáles López, I. Remote sensing and assessment of coral reef coverage at Archipiélago Espíritu Santo National Park, BCS. Kalpa Publ. Comput. 2019, 13, 56–61. [Google Scholar] [CrossRef]
- Jiménez-Illescas, Á.R.; Obeso-Nieblas, M.; Salas-de León, D.A. Oceanografía física de la Bahía de La Paz, B.C.S. In La Bahía de La Paz, Investigación y Conservación, 1st ed.; Urban, R.J., Ramírez, R.M., Eds.; Universidad Autónoma de Baja California Sur, Centro Interdisciplinario de Ciencias Marinas, SCRIPPS Institution of Oceanography: La Paz, Mexico, 1997; pp. 31–41. [Google Scholar]
- NASA Ocean Biology Processing Group. Available online: https://oceancolor.gsfc.nasa.gov/l3/ (accessed on 1 September 2021).
- Beier, E. A Numerical Investigation of the Annual Variability in the Gulf of California. J. Phys. Oceanogr. 1997, 27, 615–632. [Google Scholar] [CrossRef]
- Marinone, S.G. A three-dimensional model of the mean and seasonal circulation of the Gulf of California. J. Geophys. Res. 2003, 108, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Santamaria-Del-Angel, E.; Alvarez-Borrego, S.; Muller-Karger, F.E. Gulf of California biogeographic regions based on coastal zone color scanner imagery. J. Geophys. Res. 1994, 99, 7411–7421. [Google Scholar] [CrossRef]
- Espinosa-Carreón, T.L.; Escobedo-Urías, D. South region of the gulf of California large marine ecosystem upwelling, fluxes of CO2 and nutrients. Environ. Dev. 2017, 22, 42–51. [Google Scholar] [CrossRef]
- Russell, F. The Medusae of the British Isles: Anthomedusae, Leptomedusae, Limnomedusae, Trachymedusae, and Narcomedusae, 1st ed.; Cambridge University Press: London, UK, 1953; pp. 1–530. [Google Scholar]
- Kramp, P.L. Synopsis of the Medusae of the World. J. Mar. Biol. Assoc. 1969, 40, 7–382. [Google Scholar] [CrossRef]
- Mendoza-Becerril, M.A.; Estrada-González, M.C.; Mazariegos-Villarreal, A.; Restrepo-Avendaño, L.; Villar-Beltrán, R.D.; Agüero, J.; Cunha, A.F. Taxonomy and diversity of Hydrozoa (Cnidaria, Medusozoa) of La Paz Bay, Gulf of California. Zootaxa 2020, 4808, 1–37. [Google Scholar] [CrossRef]
- Millard, N.A.H. Monograph on the Hydroida of Southern Africa. Ann. S. Afr. Mus. 1975, 68, 1–513. [Google Scholar]
- Calder, D.R. Shallow-Water Hydroids of Bermuda the Athecatae, 1st ed.; Royal Ontario Museum: Toronto, ON, Canada, 1988; pp. 1–107. [Google Scholar] [CrossRef] [Green Version]
- Calder, D.R. Shallow-Water Hydroids of Bermuda the Thecatae, Exclusive of Plumularioidea, 1st ed.; Royal Ontario Museum: Toronto, ON, Canada, 1991; pp. 1–140. [Google Scholar] [CrossRef]
- Calder, D.R. Shallow-Water Hydroids of Bermuda Superfamily Plumularioidea, 1st ed.; Royal Ontario Museum: Toronto, ON, Canada, 1997; pp. 1–86. [Google Scholar]
- Totton, A.K.; Bargmann, H.E. A Synopsis of the Siphonophora, 1st ed.; British Museum (Natural History): London, UK, 1965; pp. 1–230. [Google Scholar]
- Palma, S.G. Contribución al estudio de los Sifonóforos encontrados frente a la costa de Valparaíso. Inv. Mar. 1973, 4, 17–88. [Google Scholar]
- Pagès, F.; Gili, J. Siphonophores (Cnidaria, Hydrozoa) of the Benguela Current (southeastern Atlantic). Sci. Mar. 1992, 56, 65–112. [Google Scholar]
- Global Invasive Species Database. Available online: www.iucngisd.org (accessed on 4 December 2022).
- CONABIO. Método de Evaluación Rápida de Invasividad (MERI) para Especies Exóticas en México; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2015; pp. 1–12. [Google Scholar]
- Estrada-González, M.C.; Agüero, J.; Mendoza-Becerril, M.A. Medusozoans from the Mexican Pacific: A review of historical and current research. J. Nat. Hist. 2022; submitted. [Google Scholar]
- Marambio, M.; Ballesteros, A.; López-Castillo, L.; Fuentes, V.; Gili, J.M. Guía de Identificación de Medusas y Otros Organismos Gelatinosos; CSIC—Instituto de Ciencias del Mar: Barcelona, Spain, 2021; pp. 1–44. [Google Scholar] [CrossRef]
- QGIS Development Team. Available online: https://www.qgis.org/es/site/ (accessed on 1 September 2021).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. Available online: https://www.R-project.org/ (accessed on 1 September 2021).
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2011, 4, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Clench, H.K. How to make regional lists of butterflies: Some thoughts. J Lepidopterists Soc. 1979, 33, 216–231. [Google Scholar]
- Soberon, J.; Llorente, J. The Use of Species Accumulation Functions for the Prediction of Species Richness. Conserv. Biol. 1993, 7, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Package ‘SpadeR’. Available online: https://cran.r-project.org/web/packages/SpadeR/SpadeR.pdf (accessed on 4 December 2022).
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Colwell, R.K.; Coddington, J.A. Estimating Terrestrial Biodiversity through Extrapolation. Philos. Trans. R. Soc. 1994, 345, 101–118. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Chiu, C. Nonparametric Estimation and Comparison of Species Richness; John Wiley & Sons, Ltd: Chichester, UK, 2016; pp. 1–11. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; pp. 1–179. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E Limited: Plymouth, UK, 2006; pp. 1–182. [Google Scholar]
- Clarke, K.R.; Somerfield, P.J.; Gorley, R.N. Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. 2008, 366, 56–69. [Google Scholar] [CrossRef]
- Package ‘vegan’. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 1 September 2021).
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in marine communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E Ltd: Plymouth, UK, 2001; pp. 1–262. [Google Scholar]
- Ihlen, V.; Zanter, K. Landsat 8 (L8) Data Users Handbook; Version 5; Landsat: Sioux Falls, SD, USA, 2019; pp. 1–106. [Google Scholar]
- The Raster Package. Available online: https://CRAN.R-project.org/package=raster (accessed on 4 December 2022).
- Rcpp: Seamless R and C++ Integration. Available online: https://cran.r-project.org/web/packages/Rcpp/index.html (accessed on 4 December 2022).
- Package ‘RStoolbox’. Available online: https://CRAN.R-project.org/package=RStoolbox (accessed on 1 September 2021).
- Package ‘rgdal’. Available online: https://cran.r-project.org/web/packages/rgdal/rgdal.pdf (accessed on 4 December 2022).
- Package, R. Nightmares. Available online: https://CRAN.R-project.org/package=nightmares (accessed on 4 December 2022).
- Matus-Hernández, M.Á.; Martínez-Rincón, R.O.; Aviña-Hernández, R.J.; Hernández-Saavedra, N.Y. Landsat-derived environmental factors to describe habitat preferences and spatiotemporal distribution of phytoplankton. Ecol. Model. 2019, 408, 108759. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.; Radin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2011, 21, 481–497. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, L.C.; Cupul-Magaña, A.L.; Rodríguez-Troncoso, A.P.; Cupul-Magaña, F.G. Distribution and species richness of caprellids (Crustacea: Amphipoda) from the Mexican Pacific. Mar. Biodivers. Rec. 2017, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Gutiérrez, J.; Funes-Rodríguez, R.; Arroyo-Ramírez, K.; Sánchez-Ortíz, C.A.; Beltrán-Castro, J.R.; Hernández-Trujillo, S.; Palomares-García, R.; Aburto-Oropeza, A.; Ezcurra, E. Oceanographic mechanisms that possibly explain dominance of neritic-tropical zooplankton species assemblages around the Islas Marías Archipelago, Mexico. Lat. Am. J. Aquat. Res. 2014, 42, 1009–1034. [Google Scholar] [CrossRef]
- SEMARNAT; CONANP. Programa de Conservación y Manejo Reserva de la Biósfera Islas Marías, 1st ed.; SEMARNAT, CONANP: Mexico City, México, 2011; pp. 1–216. [Google Scholar]
- Ajala-Batista, L.; de Miranda Lins, D.; Haddad, M.A. Diversity of estuarine and marine hydroids (Cnidaria, Hydrozoa) from subtropical ecosystems of Brazil. Mar. Biodivers. 2020, 50, 97. [Google Scholar] [CrossRef]
- Gates, A.; Horton, T.; Sepell-Stevens, A.; Chandler, C.; Grange, L.J.; Robert, K.; Bevan, A.; Jones, D.O.B. Ecological Role of an Offshore Industry Artificial Structure. Front. Mar. Sci. 2019, 6, 675. [Google Scholar] [CrossRef] [Green Version]
- Martell, L.; Bracale, R.; Carrion, S.A.; Giangrande, A.; Purcell, J.E.; Lezzi, M.; Piraino, S.; Boero, F. Successional dynamics of marine fouling hydroids (Cnidaria: Hydrozoa) at a finfish aquaculture facility in the Mediterranean Sea. PLoS ONE 2018, 13, e0196883. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Becerril, M.A.; Simões, N.; Genzano, G. Benthic hydroids (Cnidaria, Hydrozoa) from Alacranes Reef, Gulf of Mexico, Mexico. Bull Mar Sci. 2017, 94, 125–142. [Google Scholar] [CrossRef]
- Schuchert, P. Survey of the family Corynidae (Cnidaria, Hydrozoa). Rev. Suisse Zool. 2001, 108, 739–878. [Google Scholar] [CrossRef]
- Schuchert, P. Revision of the European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Families Oceanidae and Pachycordylidae. Rev. Suisse Zool. 2004, 111, 315–369. [Google Scholar] [CrossRef]
- Moura, C.J.; Cunha, M.R.; Porteiro, F.M.; Rogers, A.D. Polyphyly and cryptic diversity in the hydrozoan families Lafoeidae and Hebellidae (Cnidaria: Hydrozoa). Invertebr. Syst. 2011, 25, 454–470. [Google Scholar] [CrossRef]
- Maggioni, D.; Galli, P.; Berumen, M.L.; Arrigoni, R.; Seveso, D.; Montano, S. Astrocoryne cabela, gen. nov. et sp. nov. (Hydrozoa:Sphaerocorynidae), a new sponge-associated hydrozoan. Invertebr. Syst. 2017, 31, 734–746. [Google Scholar] [CrossRef]
- Miglietta, M.P.; Maggioni, D.; Matsumoto, Y. Phylogenetics and species delimitation of two hydrozoa (phylum Cnidaria): Turritopsis (McCrady, 1857) and Pennaria (Goldfuss, 1820). Mar. Biodivers. 2019, 49, 1085–1100. [Google Scholar] [CrossRef]
- Gueroun, S.K.M.; Piraino, S.; Yahia, O.K.; Yahia, M.N.D. Jellyfish diversity, trends and patterns in Southwestern Mediterranean Sea: A citizen science and field monitoring alliance. J. Plankton Res. 2022, 44, 819–837. [Google Scholar] [CrossRef]
- Cornelius, P.F.S. The hydroid species of Obelia (Coelenterata, Hydrozoa: Campanulariidae), with notes on the medusa stage. Bull. Br. Mus. Nat. Hist. Zool. Suppl. 1975, 28, 249–293. [Google Scholar] [CrossRef]
- CONABIO Análisis de Riesgo Rápido de Obelia dichotoma. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=117386 (accessed on 1 September 2021).
- Haydar, D. What is natural? The scale of cryptogenesis in the North Atlantic Ocean. Divers. Distrib. 2012, 18, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Calder, D.R. On a collection of hydroids (Cnidaria, Hydrozoa) from the southwest coast of Florida, USA. Zootaxa 2019, 4689, 1–141. [Google Scholar] [CrossRef] [Green Version]
- Moura, C.J.; Harris, D.J.; Cunha, M.R.; Rogers, A.D. DNA barcoding reveals cryptic diversity in marine hydroids (Cnidaria, Hydrozoa) from coastal and deep-sea environments. Zool. Scr. 2008, 37, 93–108. [Google Scholar] [CrossRef]
- Miglietta, M.P.; Odegard, D.; Faure, B.; Faucci, A. Barcoding Techniques Help Tracking the Evolutionary History of the Introduced Species Pennaria disticha (Hydrozoa, Cnidaria). PLoS ONE 2015, 10, e0144762. [Google Scholar] [CrossRef]
- Miglietta, M.P.; Lessios, H.A. A silent invasion. Biol. Invasions 2009, 11, 825–834. [Google Scholar] [CrossRef]
- Song, X.; Lyu, M.; Bernhard, R.; Wang, J.; Gravili, C. Unexpected systematic affinities and geographic expansion of a marine alien hydroid (Cnidaria:Hydrozoa). Syst. Biodivers. 2019, 17, 230–244. [Google Scholar] [CrossRef]
- Espino, F.; Otero-Ferrer, F.J.; Bosch, N.E.; Coca, J.; Haroun, R.; Tuya, F. Widespread demographic explosion of a non-indigenous hydrozoan on an oceanic island. Sci. Mar. 2020, 84, 111–118. [Google Scholar] [CrossRef]
- Carlton, J.T. Biological Invasions and Cryptogenic Species. Ecology 1996, 77, 1653–1655. [Google Scholar] [CrossRef]
- Carlton, J.T. Global change and biological invasions in the oceans. In Invasive Species in a Changing World, 1st ed.; Mooney, H.A., Hobbs, R.J., Eds.; Island Press: Washington, DC, USA, 2000; pp. 31–53. [Google Scholar]
- Ortega-Rubio, A.; González López, I.; March Mifsut, I.; Bustamante Moreno, E.I.; Palmeros Rodríguez, M.Á.; Bojórquez-Galeana, N.; Suárez, A.; Bermúnez Almada, B. Parque Nacional Zona Marina del Archipiélago Espíritu Santo: Primer Área Natural Protegida de México con el Certificado Lista Verde de UICN. Áreas Nat. Prot. Scr. 2019, 5, 43–68. [Google Scholar] [CrossRef]
- Calder, D.R.; Vervoort, W.; Hochberg, F.G. Lectotype designations of new species of hydroids (Cnidaria, Hydrozoa), described by C.M. Fraser, from Allan Hancock Pacific and Caribbean Sea Expeditions. Zool. Med. Leiden 2009, 83, 919–1058. [Google Scholar]
- Estrada-González, M.C.; Carral-Murrieta, C.O.; Molina Alonso, A.; Medina Cota, D.G.; Rosales Catalán, L.; Mendoza Becerril, M.A. Conociendo a los medusozoos de la Bahía de La Paz. In Medusozoos de la Bahía de La Paz, 1st ed.; Mendoza-Becerril, M.A., Estrada-González, M.C., Agüero, J., Eds.; Peredo y Asociados: La Paz, Mexico, 2022; pp. 42–82. [Google Scholar]
- Herrera-Cervantes, H. Sea surface temperature, ocean color and wind forcing patterns in the Bay of La Paz, Gulf of California: Seasonal variability. Atmósfera 2009, 32, 25–38. [Google Scholar] [CrossRef]
- Ulate, K.; Sánchez, C.; Sánchez-Rodríguez, A.; Alonso, D.; Aburto-Oropeza, O.; Huate-Soberanis, L. Latitudinal regionalization of epibenthic macroinvertebrate communities on rocky reefs in the Gulf of California. Mar. Biol. Res. 2016, 12, 389–401. [Google Scholar] [CrossRef]
- Robles-Tamayo, C.M.; García-Morales, R.; Valdez-Holguín, J.E.; Figueroa-Preciado, G.; Herrera-Cervantes, H.; López-Martínez, J.; Enríquez-Ocaña, L.F. Chlorophyll a concentration distribution on the mainland coast of the Gulf of California, Mexico. Remote Sens. 2020, 12, 1335. [Google Scholar] [CrossRef] [Green Version]
- Calder, D.R.; Kirkendale, L. Hydroids (Cnidaria, Hydrozoa) from Shallow-water Environments along the Caribbean Coast of Panama. Caribb. J. Sci. 2005, 41, 476–491. [Google Scholar]
- Calder, D.R. Some anthoathecate hydroids and limnopolyps (Cnidaria, Hydrozoa) from the Hawaiian archipelago. Zootaxa 2010, 2590, 1–91. [Google Scholar] [CrossRef]
- Aguirre Hinojosa, E.; Bückle Ramírez, L.F. Settlement and growth of the mussel Modiolus capax (Conrad) (Bivalvia-Mytilidae) on Artificial substrates on Bahía de los Angeles, Baja California, Mexico. Cienc. Mar. 1992, 18, 33–48. [Google Scholar] [CrossRef]
- Palomares-García, R.; Martínez-López, A.; Gárate-Lizárraga, I. Plankton community changes in Bahía Concepción, Mexico. Oceánides 2002, 17, 113–128. [Google Scholar]
- Gamero-Mora, E.; Ceballos-Corona, G.; Gasca, R.; Morales-Blake, A. Análisis de la comunidad del zooplancton gelatinoso (Hydrozoa, Ctenophora, Thaliacea) en el Pacífico central mexicano, abril-mayo 2011. Rev. Biol. Mar. Oceanogr. 2015, 50, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Silveyra-Bustamante, A.A.; Gómez-Gutiérrez, J.G.; González-Rodríguez, E.; Sánchez, C.; Schiariti, A.; Mendoza-Becerril, M.A. Seasonal variability of gelatinous zooplankton during an anomalously warm year at Cabo Pulmo National Park, Mexico. Lat. Am. J. Aquat. Res. 2020, 48, 779–793. [Google Scholar] [CrossRef]
- Alvarez-León, R.; Wedler, E. Hidroides de tres esteros adyacentes a Mazatlán, costa noroeste de México. Bol. Investig. Mar. Costeras 1982, 12, 19–32. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoglu, M.; Öztürk, B.; Can, A. New records of alien species on the Levantine coast of Turkey. Aquat. Invasions 2006, 1, 84–90. [Google Scholar] [CrossRef]






| ID | Sampling Site | Latitude (N) | Longitude (W) | Environment | Depth (m) |
|---|---|---|---|---|---|
| S01 | Los Islotes | 24°35′50″ | 110°35′50″ | Pelagic | 0–0.3 |
| S02 | Ensenada Grande | 24°33′55″ | 110°24′13″ | Pelagic | 0–0.3 |
| S03 | El Cardonal | 24°33′00″ | 110°22′55″ | Pelagic | 0–0.3 |
| S04 | Anegada de la Partida | 24°31′56″ | 110°23′39″ | Pelagic | 0–0.3 |
| 24°31′56″ | 110°23′39″ | Benthic | 0–2 | ||
| S05 | Caleta la Partida | 24°31′47″ | 110°23′00″ | Pelagic | 0–0.3 |
| S06 | El Candelero | 24°30′17″ | 110°23′30″ | Pelagic | 0–0.3 |
| S07 | Isla Ballena | 24°29′07″ | 110°23′41″ | Pelagic | 0–0.3 |
| 24°29′07″ | 110°23′52″ | Benthic | 0–2 | ||
| 24°28′43″ | 110°23′51″ | Benthic | 18 | ||
| S08 | Barco Fang-Ming | 24°26′48″ | 110°22′51″ | Pelagic | 0–0.3 |
| S09 | Bahía San Gabriel | 24°25′46″ | 110°21′52″ | Pelagic | 0–0.3 |
| 24°25′46″ | 110°21′52″ | Benthic | 0–2 | ||
| 24°25′00″ | 110°22′00″ | Benthic | ND | ||
| S10 | Punta Dispensa | 24°24′22″ | 110°21′13″ | Pelagic | 0–0.3 |
| S11 | Punta Lupona | 24°24′10″ | 110°19′31″ | Pelagic | 0–0.3 |
| S12 | Bahía Salinita | 24°28′27″ | 110°17′19″ | Pelagic | 0–0.3 |
| 24°28′27″ | 110°17′19″ | Benthic | 11.4–14.8 |
| Taxa | Life Phase | Life Stage | Biogeographic Status | Sting Level | Ecosystem Services |
|---|---|---|---|---|---|
| Class Hydrozoa Owen, 1843 | |||||
| Subclass Hydroidolina Collins, 2000 | |||||
| Superorder “Anthoathecata” Cornelius, 1992 | |||||
| “Anthoathecata” sp. Indet. | Medusa | Pelagic | NA | NA | NA |
| Bougainvillia muscus (Allman, 1863) | Medusa | Pelagic | Native | ND | ND |
| Capitata sp. Indet. | Medusa | Pelagic | NA | NA | NA |
| Corydendrium parasiticum (Linnaeus, 1767) | Polyp | Benthic | Native | ND | ND |
| Corymorpha nutans M. Sars, 1835 | Medusa | Pelagic | Cryptogenic | ND | ND |
| Corynidae sp. Indet | Polyp | Benthic | NA | NA | NA |
| Pennaria disticha Goldfuss, 1820 | Polyp | Benthic | Cryptogenic | Very stinging | ND |
| Turritopsis sp. | Polyp | Benthic | NA | NA | NA |
| Sphaerocoryne sp. | Polyp | Benthic | NA | NA | NA |
| Stauridiosarsia ophiogaster (Haeckel, 1879) | Medusa | Pelagic | Native | ND | ND |
| Superorder Leptothecata Cornelius, 1992 | |||||
| Aglaophenia pinguis Fraser, 1938 | Polyp | Benthic | Cryptogenic | Stinging | ND |
| Antennella secundaria (Gmelin, 1791) | Polyp | Benthic | Native | ND | ND |
| Clytia hemisphaerica (Linnaeus, 1767) | Medusa | Pelagic | Cryptogenic | ND | Yes 1 |
| Clytia linearis (Thornely, 1900) | Polyp | Benthic | Cryptogenic | ND | ND |
| Clytia simplex (Browne, 1902) | Medusa | Pelagic | Cryptogenic | ND | ND |
| Dynamena disticha (Bosc, 1802) | Polyp | Benthic | Native | ND | ND |
| Dynamena quadridentata (Ellis and Solander, 1786) | Polyp | Benthic | Native | ND | ND |
| Eucheilota comata (Bigelow, 1909) | Medusa | Pelagic | Native | ND | ND |
| Eucheilota paradoxica Mayer, 1900 | Medusa | Pelagic | Cryptogenic | ND | ND |
| Halopteris violae Calder, Mallinson, Collins and Hickman, 2003 | Polyp | Benthic | Cryptogenic | ND | ND |
| Hebellidae sp. Indet. | Polyp | Benthic | NA | NA | NA |
| Hydrodendron mirabile (Hincks, 1866) | Polyp | Benthic | NA | NA | NA |
| Laodicea sp. | Medusa | Pelagic | NA | NA | NA |
| Leptothecata sp. Indet. | Medusa | Belagic | NA | NA | NA |
| Macrorhynchia philippina Kirchenpauer, 1872 | Polyp | Benthic | Native | Very stinging | Yes 2 |
| Obelia dichotoma (Linnaeus, 1758) | Polyp | Benthic | Exotic | ND | ND |
| Obelia spp. | Medusa | Pelagic | NA | NA | NA |
| Plumularia floridana Nutting, 1900 | Polyp | Benthic | Cryptogenic | ND | ND |
| Ventromma halecioides (Alder, 1859) | Polyp | Benthic | Cryptogenic | ND | ND |
| Superorder Siphonophorae Eschscholtz, 1829 | |||||
| Abylopsis eschscholtzii (Huxley, 1859) | Siphonophora | Pelagic | Native | ND | Yes 3 |
| Agalmatidae sp. Indet. | Siphonophora | Pelagic | NA | ND | NA |
| Diphyes bojani (Eschscholtz, 1825) | Siphonophora | Pelagic | Native | ND | ND |
| Diphyidae sp. Indet. | Siphonophora | Pelagic | NA | ND | NA |
| Enneagonum hyalinum Quoy and Gaimard, 1827 | Siphonophora | Pelagic | Native | ND | ND |
| Eudoxoides mitra (Huxley, 1859) | Siphonophora | Pelagic | Native | ND | ND |
| Halistemma rubrum (Vogt, 1852) | Siphonophora | Pelagic | Native | ND | ND |
| Muggiaea atlantica Cunningham, 1892 | Siphonophora | Pelagic | Native | ND | ND |
| Nanomia bijuga (Delle Chiaje, 1844) | Siphonophora | Pelagic | Native | ND | ND |
| Subclass Trachylinae Haeckel, 1879 | |||||
| Aglaura hemistoma Péron and Lesueur, 1810 | Medusa | Pelagic | Native | ND | ND |
| Liriope tetraphylla (Chamisso and Eysenhardt, 1821) | Medusa | Pelagic | Native | Very stinging | Yes 4 |
| Rhopalonema velatum Gegenbaur, 1857 | Medusa | Pelagic | Native | ND | ND |
| Solmundella bitentaculata (Quoy and Gaimard, 1833) | Medusa | Pelagic | Native | ND | ND |
| Data Origin | Environmental Factor | Low Diversity | Medium Diversity | High Diversity |
|---|---|---|---|---|
| In situ | Salinity | 32.63 (±1.19) | 33.25 (±0.34) | 33.70 (±0.87) |
| Sea surface temperature (°C) | 23.21 (±0.40) | 24.04 (±0.92) | 22.97 (±1.11) | |
| Satellite | Chlorophyll-a (µg L−1) | 3.07 (±0.13) | 3.08 (±0.07) | 3.04 (±0.06) |
| Dissolved oxygen (mg L−1) | 6.09 (±0.14) | 5.80 (±0.19) | 5.73 (±0.10) | |
| Particulate organic carbon (mg m−3) | 123.40 (±3.80) | 126.67 (±8.69) | 138.05 (±31.04) | |
| pH | 8.07 (±0.19) | 8.06 (±0.04) | 7.98 (±0.02) | |
| Salinity | 34.03 (±2.08) | 36.22 (±3.20) | 34.04 (±2.13) | |
| Sea surface temperature (°C) | 17.94 (±1.39) | 20.05 (±1.50) | 20.63 (±0.79) |
| Medium | High | Low × Medium | Low × High | Medium × High | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Av.Sim | Sim/SD | Contri.% | Av.Sim | Sim/SD | Contri.% | Av.Diss | Diss/SD | Contri.% | Av.Diss | Diss/SD | Contri.% | Av.Diss | Diss/SD | Contri.% |
| Macrorhynchia philippina | - | - | - | 7.40 | 3.70 | 14.07 | - | - | - | 6.61 | 2.88 | 7.60 | 5.46 | 2.91 | 6.76 |
| Obelia dichotoma | - | - | - | 7.40 | 3.70 | 14.07 | - | - | - | 6.61 | 2.88 | 7.60 | 5.46 | 2.91 | 6.76 |
| Clytia linearis | - | - | - | 7.40 | 3.70 | 14.07 | - | - | - | 6.61 | 2.88 | 7.60 | 4.21 | 1.38 | 5.21 |
| Stauridiosarsia ophiogaster | 18.20 | 2.93 | 42.52 | - | - | -- | 13.82 | 1.89 | 16.49 | - | - | - | 5.46 | 2.91 | 6.76 |
| Abylopsis eschscholtzii | 18.20 | 2.93 | 42.52 | - | - | - | 13.82 | 1.89 | 16.49 | - | - | - | 4.41 | 1.41 | 5.45 |
| Muggiaea atlantica | - | - | - | - | - | - | 7.37 | 1.02 | 8.80 | - | - | - | 3.3 | 1.11 | 4.09 |
| Hydrodendron mirabile | - | - | - | - | - | - | - | - | - | 5.40 | 1.41 | 6.21 | 4.41 | 1.41 | 5.45 |
| Pennaria disticha | - | - | - | - | - | - | - | - | - | 5.40 | 1.41 | 6.21 | 4.41 | 1.41 | 5.45 |
| Dynamena disticha | - | - | - | - | - | - | - | - | - | 4.44 | 1.36 | 5.11 | 3.73 | 1.39 | 4.62 |
| Aglaophenia pinguis | - | - | - | - | - | - | - | - | - | 4.44 | 1.36 | 5.11 | 3.26 | 1.10 | 4.03 |
| Plumularia floridana | - | - | - | - | - | - | - | - | - | 4.44 | 1.36 | 5.11 | 3.26 | 1.10 | 4.03 |
| Ventromma halecioides | - | - | - | - | - | - | - | - | - | 4.44 | 1.36 | 5.11 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-González, M.C.; Jiménez-López, M.E.; Huato-Soberanis, L.; Mendoza-Becerril, M.A. Socioenvironmental and Spatial Criteria as Tools for the Management and Conservation of Hydrozoans in Protected and Unprotected Areas. Diversity 2023, 15, 182. https://doi.org/10.3390/d15020182
Estrada-González MC, Jiménez-López ME, Huato-Soberanis L, Mendoza-Becerril MA. Socioenvironmental and Spatial Criteria as Tools for the Management and Conservation of Hydrozoans in Protected and Unprotected Areas. Diversity. 2023; 15(2):182. https://doi.org/10.3390/d15020182
Chicago/Turabian StyleEstrada-González, Mariae C., María Esther Jiménez-López, Leonardo Huato-Soberanis, and María A. Mendoza-Becerril. 2023. "Socioenvironmental and Spatial Criteria as Tools for the Management and Conservation of Hydrozoans in Protected and Unprotected Areas" Diversity 15, no. 2: 182. https://doi.org/10.3390/d15020182
APA StyleEstrada-González, M. C., Jiménez-López, M. E., Huato-Soberanis, L., & Mendoza-Becerril, M. A. (2023). Socioenvironmental and Spatial Criteria as Tools for the Management and Conservation of Hydrozoans in Protected and Unprotected Areas. Diversity, 15(2), 182. https://doi.org/10.3390/d15020182