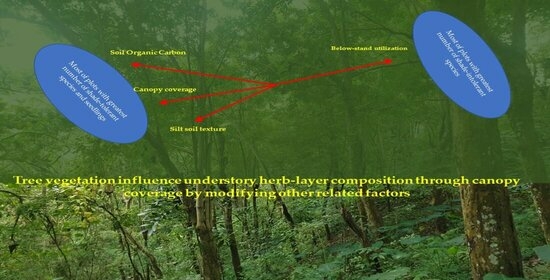
Role of Tree Vegetation and Associated Environmental Factors on the Understory Herb-Layer Composition in a Reforested Area: A Study from “Kulon Progo Community Forestry”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.3. Data Arrangement
2.4. Statistical Analysis
3. Results
3.1. Effect of Stand Type on Understory Herb-Layer Composition
3.2. Influencing Factors Determining the Understory Herb-Layer Composition
3.2.1. Control of Tree Stand Characteristics and Associated Environmental Factors on Understory Species
3.2.2. Inter-Correlation between Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No | Species Name | Family Name | Important Value Index | Shading | Distribution | ||||
|---|---|---|---|---|---|---|---|---|---|
| PN | AS | SA | MA | TD | Characteristic | Category | |||
| 1 | Dryopteris sp. | Dryopteridaceae | 7.33 | 5.77 | 4.48 | 3.54 | 4.56 | 1 | 1 |
| 2 | Cytrococum | Poaceae | 23.81 | 17.29 | 24.88 | 24.75 | 3.02 | 2 | 1 |
| 3 | Ottochloa | Poaceae | 6.35 | 4.74 | 1.38 | 4.73 | 26.89 | 1 | 1 |
| 4 | Curcuma sp. | Zingeberacerae | 2.25 | 4.53 | 0.00 | 2.90 | 1.10 | 3 | 1 |
| 5 | Urena lobata | Malvaceae | 1.48 | 1.25 | 2.37 | 5.06 | 1.85 | 2 | 1 |
| 6 | Clidemia hirta | Melastomaceae | 6.35 | 2.72 | 6.59 | 5.26 | 4.39 | 3 | 1 |
| 7 | Borreria latifolia | Rubiaceae | 1.41 | 0.00 | 0.00 | 0.88 | 0.00 | 1 | 2 |
| 8 | Elletaria cardomomum | Zingeberacerae | 1.41 | 0.00 | 0.00 | 0.00 | 0.00 | 2 | 3 |
| 9 | Impatiens balsamina | Balsaminaceae | 1.48 | 0.00 | 0.00 | 0.00 | 0.00 | 2 | 3 |
| 10 | Andrographis paniculata | Acanthaceae | 3.10 | 5.09 | 2.46 | 0.00 | 6.99 | 1 | 2 |
| 11 | Colocasia gigantea | Araceae | 1.62 | 2.26 | 0.73 | 0.00 | 0.00 | 2 | 2 |
| 12 | Slaginella sp. | Slaginellaceae | 9.31 | 4.97 | 0.00 | 2.63 | 0.00 | 2 | 2 |
| 13 | Coctus spicatus | Costaceae | 1.69 | 1.36 | 0.73 | 0.74 | 0.00 | 2 | 2 |
| 14 | Scleria sp. | Poaceae | 1.90 | 0.00 | 0.00 | 4.99 | 0.86 | 3 | 2 |
| 15 | Euphorbia hirta | Euphorbiaceae | 0.92 | 3.17 | 1.55 | 0.00 | 0.00 | 3 | 2 |
| 16 | Eupatorium odoratum | Asteraceae | 5.42 | 0.00 | 2.13 | 0.81 | 1.58 | 3 | 2 |
| 17 | Oxalis corniculata | Oxalidaceae | 0.63 | 1.47 | 0.82 | 0.00 | 0.74 | 2 | 2 |
| 18 | Stachytarpheta jamaicensis | Verbenaceae | 0.00 | 0.00 | 1.73 | 1.82 | 0.00 | 3 | 2 |
| 19 | Spilanthes paniculata | Asteraceae | 3.45 | 0.00 | 0.00 | 0.00 | 0.00 | 3 | 3 |
| 20 | Pennisetum purpureum | Poaceae | 0.63 | 0.00 | 2.30 | 2.90 | 1.46 | 2 | 2 |
| 21 | Commelina benghalensis | Clommelinaceae | 0.70 | 0.00 | 0.00 | 0.00 | 0.00 | 3 | 3 |
| 22 | Lantana camara | Verbenaceae | 0.70 | 1.70 | 1.10 | 4.99 | 1.61 | 2 | 2 |
| 23 | Setaria viridis | Poaceae | 1.13 | 0.00 | 0.00 | 0.00 | 0.00 | 3 | 3 |
| 24 | Phylanthus urinaria | Fabaceae | 0.00 | 1.15 | 0.82 | 3.17 | 0.00 | 3 | 2 |
| 25 | Acalypha australis | Euphorbiaceae | 1.69 | 0.00 | 0.00 | 0.00 | 0.00 | 3 | 3 |
| 26 | Imperata cylindrica | Poaceae | 1.27 | 0.00 | 0.00 | 0.00 | 0.00 | 3 | 3 |
| 27 | Centrosema sp. | Fabaceae | 0.63 | 5.66 | 3.38 | 3.51 | 4.56 | 2 | 1 |
| 28 | Passiflota foetida | Passifloraceae | 0.00 | 0.91 | 0.00 | 0.00 | 0.00 | 2 | 3 |
| 29 | Ficus montana | Moraceae | 0.00 | 1.81 | 0.00 | 0.00 | 0.00 | 3 | 3 |
| 30 | Almorphophallus muelleri | Araceae | 0.00 | 0.91 | 0.00 | 0.00 | 0.00 | 2 | 3 |
| 31 | Panicum repens | Poaceae | 0.00 | 2.15 | 0.00 | 0.88 | 0.00 | 2 | 2 |
| 32 | Melastoma cadidum | Melastomaceae | 0.00 | 2.26 | 1.76 | 2.16 | 1.97 | 3 | 2 |
| 33 | Ageratum conyzoides | Asteraceae | 0.00 | 7.57 | 2.84 | 8.91 | 3.19 | 3 | 2 |
| 34 | Mimosa pudica | Fabaceae | 0.00 | 1.25 | 0.00 | 0.00 | 0.00 | 3 | 3 |
| 35 | Elephantopus scaber | Asteraceae | 0.00 | 1.93 | 1.10 | 0.00 | 0.00 | 2 | 2 |
| 36 | Cymbopogon citratus | Poaceae | 0.00 | 1.92 | 1.01 | 2.29 | 0.00 | 2 | 2 |
| 37 | Flemingia macrophylla | Fabaceae | 0.00 | 0.00 | 0.00 | 1.15 | 0.86 | 2 | 2 |
| 38 | Hyptis capitata | Lamiaceae | 0.00 | 2.26 | 1.83 | 0.00 | 0.00 | 3 | 2 |
| 39 | Stenochlaena palustris | Blechnaceae | 0.00 | 0.00 | 2.49 | 0.00 | 0.00 | 2 | 3 |
| 40 | Hibiscus rosa-sinensis | Malvaceae | 0.00 | 0.00 | 0.82 | 0.00 | 0.00 | 2 | 3 |
| 41 | Cynodon dactylon | Poaceae | 0.00 | 0.00 | 10.22 | 0.00 | 0.98 | 3 | 2 |
| 42 | Mikania micrantha | Asteraceae | 0.00 | 0.00 | 2.20 | 0.00 | 0.00 | 3 | 3 |
| 43 | Tinospora cordifolia | Menispermaceae | 0.00 | 0.00 | 0.00 | 0.00 | 1.85 | 1 | 3 |
| 44 | Pteris ensiformis | Pteridaceae | 0.00 | 0.00 | 1.10 | 0.00 | 0.00 | 1 | 3 |
| 45 | Cosmos caudatus | Coreopsideae | 0.00 | 0.00 | 0.91 | 0.00 | 0.00 | 1 | 3 |
| 46 | Gynura procumbens | Asteraceae | 0.00 | 0.00 | 0.00 | 0.88 | 0.00 | 2 | 3 |
| 47 | Cynoglosum sp. | Boraginaceae | 0.00 | 0.00 | 1.01 | 0.00 | 0.00 | 1 | 3 |
| 48 | Wedelia trilobata | Asteraceae | 0.00 | 0.00 | 1.29 | 0.00 | 1.22 | 3 | 2 |
| 49 | Peperonema pellucida | Piperaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.74 | 1 | 3 |
| 50 | Lygodium circinnatum | Lygodiaceae | 0.00 | 0.00 | 0.00 | 1.62 | 2.35 | 2 | 2 |
| 51 | Portulaca sp. | Portulacaceae | 0.00 | 0.00 | 0.82 | 0.00 | 0.00 | 3 | 3 |
| 52 | Andiatum caudatum | Andiataceae | 0.00 | 0.00 | 0.00 | 0.74 | 0.00 | 2 | 3 |
| 53 | Crassocephalum crepidioides | Asteraceae | 0.00 | 0.00 | 0.00 | 0.74 | 0.86 | 3 | 2 |
| 54 | Clausena excavata | Rutaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.74 | 2 | 3 |
| 55 | Bauhinia sp. | Fabaceae | 0.00 | 0.00 | 0.00 | 0.00 | 4.80 | 1 | 3 |
| 56 | Cleome rutidosperma | Cleomaceae | 0.00 | 1.47 | 0.00 | 0.00 | 0.86 | 2 | 2 |
| 57 | Clitoria ternatea | Fabaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.86 | 2 | 3 |
| 58 | Nephrolepis bisserata | Pteridacerae | 0.00 | 0.00 | 0.00 | 0.00 | 2.71 | 2 | 3 |
| 59 | Syzigium aromaticum | Myrtaceae | 0.70 | 0.00 | 0.00 | 0.00 | 0.00 | 2 | 3 |
| 60 | Pinus merkusii * | Pinaceae | 0.92 | 0.00 | 0.00 | 0.00 | 0.00 | 2 | 3 |
| 61 | Macaranga sp. * | Euphorbiaceae | 2.39 | 1.00 | 1.73 | 2.70 | 1.85 | 2 | 2 |
| 62 | Dalbergia latifolia * | Fabaceae | 2.95 | 0.00 | 2.65 | 2.43 | 5.04 | 1 | 2 |
| 63 | Swietenia macrophyla * | Meliaceae | 0.63 | 6.68 | 3.47 | 0.74 | 3.10 | 1 | 1 |
| 64 | Gmelina elliptica * | Lamiaceae | 0.00 | 0.00 | 0.00 | 0.00 | 4.08 | 2 | 2 |
| 65 | Tectona grandis * | Lamiaceae | 0.00 | 0.91 | 0.00 | 0.00 | 1.49 | 3 | 2 |
| 66 | Ardisi elliptica | Primulaceae | 4.50 | 2.04 | 1.92 | 0.00 | 0.74 | 1 | 2 |
| 67 | Parkia speciosa * | Fabaceae | 0.00 | 0.00 | 1.10 | 0.00 | 0.00 | 2 | 3 |
| 68 | Genetum genemon * | Genetaceae | 0.00 | 0.91 | 0.00 | 0.00 | 0.00 | 1 | 3 |
| 69 | Ficus septica * | Moraceae | 1.27 | 0.91 | 0.73 | 0.00 | 0.00 | 1 | 2 |
| 70 | Acacia auriculiformis * | Fabaceae | 0.00 | 0.00 | 0.82 | 0.74 | 0.00 | 1 | 2 |
| 71 | Leucaena leucocephala * | Fabaceae | 0.00 | 0.00 | 0.73 | 0.00 | 0.00 | 2 | 3 |
| 72 | Paraserientes falcataria * | Fabaceae | 0.00 | 0.00 | 0.00 | 1.35 | 0.00 | 2 | 3 |
| 100 | 100 | 100 | 100 | 100 | |||||
| Stand Type | Tree Regeneration Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Seedling Species | Species | Family | Density (n/ha) | RS | ||||
| Seedling | Sapling | Pole | Tree | |||||
| PN | 2 | P. merkusii | Lauraceae | 1786 | - | 14 | 311 | |
| Other species | 14,285 | 886 | 186 | 164 | Good | |||
| Total | 16,071 | 886 | 200 | 375 | Good | |||
| AS | 1 | A. molucana | Euphorbiaceae | - | - | - | 104 | None |
| S. macrophylla | Euphorbiaceae | 7188 | 343 | 167 | 32 | Good | ||
| Other species | 3512 | 685 | 176 | 68 | ||||
| Total | 10,700 | 1028 | 343 | 204 | Good | |||
| SA | 2 | S. macrophylla | Moraceae | 1875 | 686 | 229 | 54 | Good |
| A. auriculiformis | Euphorbiaceae | 2083 | 171 | 14 | 54 | Good | ||
| Other species | 9242 | 985 | 285 | 124 | Good | |||
| Total | 13,200 | 1842 | 528 | 232 | Good | |||
| MA | 1 | M. Leucadendron | Moraceae | - | - | 186 | 79 | None |
| A. auriculiformis | Euphorbiaceae | 682 | 29 | 67 | 36 | Good | ||
| Other species | 7868 | 1113 | 147 | 36 | Good | |||
| Total | 8550 | 1142 | 400 | 154 | Good | |||
| TD | 2 | D. latifolia | Moraceae | 3958 | 514 | 167 | 118 | Good |
| T. grandis | Euphorbiaceae | - | 57 | 67 | 74 | Poor | ||
| Other species | 5684 | 400 | 195 | 19 | Good | |||
| Total | Euphorbiaceae | 9642 | 971 | 429 | 211 | Good | ||
References
- Nilsson, M.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Jules, M.J.; Sawyer, J.O.; Jules, E.S. Assessing the relationships between stand development and understory vegetation using a 420-year chronosequence. For. Ecol. Manag. 2008, 255, 2384–2393. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Hubau, W.; De Mil, T.; Van den Bulcke, J.; Phillips, O.L.; Angoboy Ilondea, B.; Van Acker, J.; Sullivan, M.J.P.; Nsenga, L.; Toirambe, B.; Couralet, C.; et al. The persistence of carbon in the African forest understory. Nat. Plants 2019, 5, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Landuyt, D.; De Lombaerde, E.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; De Frenne, P.; Ma, S.; Proesmans, W.; Blondeel, H. The functional role of temperate forest understorey vegetation in a changing world. Glob. Chang. Biol. 2019, 25, 3625–3641. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y.H. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef]
- Dauber, J.; Hirsch, M.; Simmering, D.; Waldhardt, R.; Otte, A.; Wolters, V. Landscape structure as an indicator of biodiversity: Matrix effects on species richness. Agric. Ecosyst. Environ. 2003, 98, 321–329. [Google Scholar] [CrossRef]
- Malik, Z.A.; Bhatt, A.B. Regeneration status of tree species and survival of their seedlings in Kedarnath Wildlife Sanctuary and its adjoining areas in Western Himalaya, India. Trop. Ecol. 2016, 57, 677–690. [Google Scholar]
- Wahyuni, I. Distribution of invasive plant species in different land-use systems in Sumatera, Indonesia. Biotropia 2016, 23, 127–135. [Google Scholar] [CrossRef]
- Fu, D.; Wu, X.; Huang, N.; Duan, C. Effects of the invasive herb Ageratina adenophora on understory plant communities and tree seedling growth in Pinus yunnanensis forests in Yunnan, China. J. For. Res. 2018, 23, 112–119. [Google Scholar] [CrossRef]
- Siswo, S.; Yun, C.W.; Kim, H.; Lee, J.; Atmoko, B.D.; Brahmantya, L. Assessing herb layer composition under jungle rubber in Sungai Manau Forest, Jambi, Indonesia: Indicator species and tree regeneration potential. Biodiversitas 2022, 23. [Google Scholar] [CrossRef]
- Beukema, H.; van Noordwijk, M. Terrestrial pteridophytes as indicators of a forest-like environment in rubber production systems in the lowlands of Jambi, Sumatra. Agric. Ecosyst. Environ. 2004, 104, 63–73. [Google Scholar] [CrossRef]
- Böhnert, T.; Wenzel, A.; Altenhövel, C.; Beeretz, L.; Tjitrosoedirdjo, S.S.; Meijide, A.; Rembold, K.; Kreft, H. Effects of land-use change on vascular epiphyte diversity in Sumatra (Indonesia). Biol. Conserv. 2016, 202, 20–29. [Google Scholar] [CrossRef]
- Udayana, C.; Andreassen, H.P.; Skarpe, C. Understory diversity and composition after planting of teak and mahogany in Yogyakarta, Indonesia. J. Sustain. For. 2020, 39, 494–510. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.F.; Gao, T.; Zhang, W.; Li, G.; Hao, W.F. Understory Vegetation Composition and Stand Are Mainly Limited by Soil Moisture in Black Locust Plantations of Loess Plateau. Forests 2021, 12, 195. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved— A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Pilon, N.A.; Durigan, G.; Rickenback, J.; Pennington, R.T.; Dexter, K.G.; Hoffmann, W.A.; Abreu, R.C.; Lehmann, C.E. Shade alters savanna grass layer structure and function along a gradient of canopy cover. J. Veg. Sci. 2021, 32, e12959. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, M.; Yin, F.; Zhang, M.-J.; Wei, J. Tree Cover Improved the Species Diversity of Understory Spontaneous Herbs in a Small City. Forests 2022, 13, 1310. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Yang, Z.; Ji, X. Vegetation succession process induced by reforestation in erosion areas. Front. For. China 2008, 3, 279–285. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [Green Version]
- Ou, Z.; Pang, S.; He, Q.; Peng, Y.; Huang, X.; Shen, W. Effects of vegetation restoration and environmental factors on understory vascular plants in a typical karst ecosystem in southern China. Sci. Rep. 2020, 10, 12011. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.A.; Chen, H.Y.H. Understory vegetation dynamics of North American boreal forests. Crit. Rev. Plant Sci. 2006, 25, 381–397. [Google Scholar] [CrossRef]
- Huo, H.; Feng, Q.; Su, Y.H. The influences of canopy species and topographic variables on understory species diversity and composition in coniferous forests. Sci. World J. 2014, 1, 252489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thammanu, S.; Marod, D.; Han, H.; Bhusal, N.; Asanok, L.; Ketdee, P.; Gaewsingha, N.; Lee, S.; Chung, J. The influence of environmental factors on species composition and distribution in a community forest in Northern Thailand. J. For. Res. 2020, 32, 649–662. [Google Scholar] [CrossRef]
- Siswo; Yun, C.-W.; Abdiyani, S. Assessing Vegetation Composition and the Indicator Species around Water Source Areas in a Pine Forest Plantation: A Case Study from Watujali and Silengkong Catchments, Kebumen, Indonesia. Forests 2019, 10, 825. [Google Scholar] [CrossRef] [Green Version]
- De Steven, D.; Faulkner, S.P.; Keeland, B.D.; Baldwin, M.J.; McCoy, J.W.; Hughes, S.C. Understory vegetation as an indicator for floodplain forest restoration in the Mississippi River Alluvial Valley, USA. Restor. Ecol. 2015, 23, 402–412. [Google Scholar] [CrossRef]
- Udayana, C.; Andreassen, H.P.; Skarpe, C. Wood and non-wood forest products of Central Java, Indonesia. J. Sustain. For. 2019, 38, 715–732. [Google Scholar] [CrossRef] [Green Version]
- Mahfudz; Rahman, T.; Lukmansjah, D. (Eds.) Statistik Kementeria n Lingkungan Hidup dan Kehutanan Tahun 2019 [Ministry of Environment and Forestry Statistics for 2019]; Kementerian Lingkungan Hidup dan kehutanan [Ministry of Environment and Forestry]: Jakarta, Indonesia, 2020. [Google Scholar]
- Nawir, A.A.; Rumboko, L. Forest Rehabilitation in Indonesia: Where to after More Than Three Decades? Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2007. [Google Scholar]
- HKm Mandiri. Profil Kelompok Tani Hutan Kemasyarakatan [Profile of Community Forest Farmer Groups]; HKm Mandiri: Yogyakarta, Indonesia, 2022. [Google Scholar]
- Siswo; Atmoko, D.A.; Brahmantya, L.; Pahlana, U.W.; Yun, C.W. Overseas Forest Survey; Tree Species Distribution and the Rela-tionship to Environmental Factors in the Protected Forest of Kulon Progo Community Forestry; Report; Kongju National University: Yesan-gun, Republic of Korea, 2022. [Google Scholar]
- Siswo; Kim, H.; Lee, J.; Yun, C.-W. Influence of Tree Vegetation and The Associated Environmental Factors on Soil Organic Carbon; Evidence from “Kulon Progo Community Forestry,” Yogyakarta, Indonesia. Forests 2023, 14, 365. [Google Scholar] [CrossRef]
- Hadi, E.E.W.; Widyastuti, S.M.; Wahyuono, S. Keanekaragaman Dan Pemanfaatan Tumbuhan Bawah Pada Sistem Agroforestri Di Perbukitan Menoreh, Kabupaten Kulon Progo [Diversity and Untilization of Understorey in Agroforestry System of Menoreh Hill, Kulon Progo Regency]. J. People Environ. 2016, 23, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Zuhri, S. Keanekaragaman Jenis Tumbuhan Bawah Pada Lahan Andil Kelompok Tani Hutan “Mandiri” di Kalibiru, Kabupaten Kulon Progo [Diversity of Understorey Species on the Utilization Permits of the “Mandiri” Forest Farmer Group in Kalibiru, Kulon Progo Regency]. Ph.D. Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2021. [Google Scholar]
- Balai KPH Yogyakarta. Ringkasan Eksekutif Rencana Pengelolaan KPH Yogykarta Jangka Tahun 2014–2023 [Executive Summary of the KPH Yogyakarta Management Plan for the 2014–2023 Term]; Balai Kesatuan Pengelolaan Hutan: Yogyakarta, Indonesia, 2013. [Google Scholar]
- Arsalan, A.; Gravitiani, E.; Irianto, H. Biomassa di Atas Tanah dan Penghitungan Simpanan Karbon Hutan Kalibiru Kabupaten Kulon Progo [Aboveground Biomass and Calculation of Carbon Stores in the Kalibiru Forest, Kulon Progo Regency]. J. Penelit. Biol. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- BPS Kabupaten Kulon Progo. Kabupaten Kulon Progo Dalam Angka Tahun 2021 [Kulon Progo Regency in Figures for 2021]; Biro Pusat Statistik: Kulon Progo, Indonesia, 2022. [Google Scholar]
- Climate Engine. Computing Climate and Remote Sensing Data. Available online: https://app.climateengine.com/climateEngine (accessed on 22 September 2022).
- Wikipedia. Sejarah Terjadinya Hutan Negara di Kulon Progo. Available online: https://id.wikipedia.org/wiki/Kalibiru (accessed on 22 September 2022).
- Kementerian Kehutanan. Keputusan Menteri Kehutanan no SK.437/Menhut-II/2007 Tentang Penetapan Areal Kerja Hutan Kemasyarakatan di Kabupaten Kulon Progo Provinsi Daerah Istimewa Yogyakarta [Decree of the Minister of Forestry no SK.437/Menhut-II/2007 Regarding the Determination of Community Forestry Areas in Kulon Progo Regency, Special Teritory of Yogyakarta Province]; Kemeterian Kehutanan [Ministry of Forestry]: Jakarta, Indonesia, 2007. [Google Scholar]
- Kusmana, C. Metode Survey Vegetasi [Vegetation Survey Method]; Institut Pertanian Bogor: Bogor, Indonesia, 1997. [Google Scholar]
- Backer, C.A.; Van-Den- Bakhuizen, B. Flora of Java (Spermatophytes Only); Nordhoff: Groningen, The Nederland, 1963. [Google Scholar]
- Yudhoyono, A.; Sukarya, D.G. 3500 Plant Species of the Botanic Gardens of Indonesia; PT. Sukarya dan Sukarya Pendetama: Jakarta, Indonesia, 2013. [Google Scholar]
- Kudo, Y.; Mutaqien, Z.; Simbolon, H.; Suzuki, E. Spread of invasive plants along trails in two national parks in West Java, Indonesia. Tropics 2014, 23, 99–110. [Google Scholar] [CrossRef] [Green Version]
- GISD 2022. Invasive Species Specialist Group. Available online: http://www.iucngisd.org/gisd/search.php (accessed on 19 January 2022).
- Setyawati, T.; Narulita, S.; Bahri, I.P.; Raharjo, G.T. A Guide Book to Invasive Alien Plant Species in Indonesia; Research, Development and Innovation Agency. Ministry of Environment and Forestry: Bogor, Indonesia, 2015. [Google Scholar]
- Dar, J.A.; Subashree, K.; Sundarapandian, S.; Saikia, P.; Kumar, A.; Khare, P.K.; Dayanadan, S.; Khan, M.L. Invasive Species and Their Impact on Tropical Forests of Central India: A Review. In Tropical Ecosystems: Structure, Functions and Challenges in the Face of Global Change; Springer: Singapore, 2019; pp. 69–109. [Google Scholar] [CrossRef]
- Sarkar, M.; Devi, A. Assessment of diversity, population structure and regeneration status of tree species in Hollongapar Gibbon Wildlife Sanctuary, Assam, Northeast India. Trop. Plant Res. 2014, 1, 26–36. [Google Scholar]
- Nelson, J.; Noweg, T. Assessment of Forest Regeneration following series of disturbances in two types of primary forest at Bungo Range, Bau, Sarawak. J. Trop. For. Sci. 2021, 33, 126–136. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MJM Software Design: Corvallis, OR, USA, 2002. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science: Malden, MA, USA, 2004. [Google Scholar]
- Peck, J.E. Multivariate Analysis for Community Ecologists; MJM Software Design: Corvallis, OR, USA, 2010. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Cardinal, R.N.; Aitken, M.R. ANOVA for the Behavioral Sciences Researcher; Psychology Press: London, UK, 2013. [Google Scholar]
- Cleophas, T.J.; Zwinderman, A.H. Non-Parametric Tests for Three or More Samples (Friedman and Kruskal Wallis); Clinical Data Analysis on a Poscket Calculator; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ter Braak, C.J.; Verdonschot, P.F. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Møller, A.; Jennions, M.D. How much variance can be explained by ecologists and evolutionary biologists? Oecologia 2022, 132, 492–500. [Google Scholar] [CrossRef]
- Abood, S.A.; Lee, J.S.H.; Burialova, Z.; Garcia-Ulloa, J.; Koh, L.P. Relative contributions of the logging, fiber, oil palm, and mining industries to forest loss in Indonesia. Conserv. Lett. 2015, 8, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Meijide, A.; Badu, C.S.; Moyano, F.; Tiralla, N.; Gunawan, D.; Knohl, A. Impact of forest conversion to oil palm and rubber plantations on microclimate and the role of the 2015 ENSO event. Agric. For. Meteorol. 2018, 252, 208–219. [Google Scholar] [CrossRef]
- Osborne, B.; Nasto, M.K.; Soper, F.M.; Asner, G.P.; Balzotti, C.S.; Cleveland, C.C.; Taylor, P.G.; Townsend, A.R.; Porder, S. Leaf litter inputs reinforce islands of nitrogen fertility in a lowland tropical forest. Biogeochemistry 2020, 147, 293–306. [Google Scholar] [CrossRef]
- Ehrlén, J.; Eriksson, O. Dispersal limitation and patch occupancy in forest herbs. Ecology 2000, 81, 1667–1674. [Google Scholar] [CrossRef]
- Ismaini, L. Pengaruh alelopati tumbuhan invasif (Clidemia hirta) terhadap germinasi biji tumbuhan asli (Impatiens platypetala) [Effect of invasive plant allelopathy (Clidemia hirta) on seed germination of native plants (Impatiens platypetala)]. Pros. Sem. Nas. Masy. Biodiv. Indon. 2015, 1, 834–837. [Google Scholar]
- Breaden, R.C.; Brooks, S.J.; Murphy, H.T. The biology of Australia weeds Clidemia hirta (L.) D.Don. Plant Prot. Q. 2012, 27, 3–18. [Google Scholar]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odum, H.T. Scales of ecological engineering. Ecol. Eng. 1996, 6, 7–19. [Google Scholar] [CrossRef]
- Su, X.; Wang, M.; Huang, Z.; Fu, S.; Chen, H.Y. Forest understorey vegetation: Colonization and the availability and heterogeneity of Resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef] [Green Version]
- Rembold, K.; Mangopo, H.; Tjitrosoedirdjo, S.S.; Kreft, H. Plant diversity, forest dependency, and alien plant invasions in tropical agricultural landscapes. Biol. Conserv. 2017, 213, 234–242. [Google Scholar] [CrossRef]
- Indriyanto. Ekologi Hutan [Forest Ecology]; Bumi Aksara: Jakarta, Indonesia, 2008. [Google Scholar]
- Aoyagi, R.; Imai, N.; Kitayama, K. Ecological significance of the patches dominated by pioneer trees for the regeneration of dipterocarps in a Bornean logged-over secondary forest. For. Ecol. Manag. 2013, 289, 378–384. [Google Scholar] [CrossRef]
- Yassir, I. Diversity of plant communities in secondary succession of Imperata grasslands in Samboja Lestari, East Kalimantan, Indonesia. Indones. J. For. Res. 2014, 1, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Freeman, C. The impact of treefall gaps on the species richness of invasive plants. J. Young Investig. 2015, 28, 1. [Google Scholar]
- Waheed, M.; Haq, S.M.; Fatima, K.; Arshad, F.; Bussmann, R.W.; Masood, F.R.; Alataway, A.; Dewidar, A.Z.; Almutairi, K.F.; Elansary, H.O.; et al. Ecological Distribution Patterns and Indicator Species Analysis of Climber Plants in Changa Manga Forest Plantation. Diversity 2022, 14, 988. [Google Scholar] [CrossRef]
- EOS Data Analytics. Type of Soil in Agriculture to Grow Crops Efficiency. Available online: https://eos.com/blog/types-of-soil/ (accessed on 19 July 2023).
- Janzen, H.H. The soil carbon dilemma: Shall we hoard it or use it? Soil Biol. Biochem. 2006, 38, 419–424. [Google Scholar] [CrossRef]
- Pereira, H.; Navarro, L.; Martins, I. Global biodiversity change: The bad, the good, and the unknown. Annu. Rev. Environ. Resour. 2012, 37, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Mu, X.; Gao, P.; Zhao, G.; Sun, W.; Tatarko, J.; Tan, X. Influence of vegetation restoration on soil physical properties in the Loess Plateau, China. J. Soils Sediments 2019, 19, 716–728. [Google Scholar] [CrossRef]
- Labrière, N.; Locatelli, B.; Laumonier, Y.; Freycon, V.; Bernoux, M. Soil erosion in the humid tropics: A systematic quantitative review. Agric. Ecosyst. Environ. 2015, 203, 127–139. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Sun, C. Soil organic carbon and total nitrogen storage as affected by land use in a small watershed of the Loess Plateau, China. Eur. J. Soil Biol. 2013, 54, 16–24. [Google Scholar] [CrossRef]
- Hu, P.L.; Liu, S.J.; Ye, Y.Y.; Zhang, W.; Wang, K.L.; Su, Y.R. Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. Land Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Chengrui, M.; Dregne, H.E. Review article: Silt and the future development of China’s Yellow River. Geogr. J. 2001, 167, 7–22. [Google Scholar] [CrossRef]
- Roose, E.J.R.; Lal, C.; Feller, B.; Barthes; Stewart, B.A. Advances in Soil Science: Soil Erosion and Carbon Dynamics; CRC Press, Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2006; Volume 352, p. 88. [Google Scholar]
- Wang, Y.; Fu, B.; Lü, Y.; Chen, L. Effects of vegetation restoration on soil organic carbon sequestration at multiple scales in semi-arid Loess Plateau, China. Catena 2011, 85, 58–66. [Google Scholar] [CrossRef]
- Wenjie, L.; Pengju, L.; Hongmei, L.; Weinping, D. Estimation of evaporation rate from soil surface using stable isotopic com-position of throughfall and stream water in a tropical seasonal rain forest of Xishuangbanna, Southwest China. Acta Ecol. Sin. 2016, 26, 1303–1310. [Google Scholar] [CrossRef]
- Fan, J.; Ostergaard, K.T.; Guyot, A.; Fujiwara, S.; Lockington, D.A. Estimating groundwater evapotranspiration by a sub-tropical pine plantation using diurnal water table fluctuations: Implication from night-time water use. J. Hydrol. 2016, 542, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, S.; Sun, X.; Yu, X. Variations of forest soil organic carbon and its influencing factors in east China. Ann. For. Sci. 2016, 73, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Devi, A.S. Influence of trees and associated variables on soil organic carbon: A review. J. Ecol. Environ. 2021, 45, 5. [Google Scholar] [CrossRef]
- Joshi, L.; Wibawa, G.; Vincent, G.; Boutin, D.; Akiefnawati, R.; Manurung, G.; Van-Noordwijk, M.; Williams, S. Jungle Rubber; World Agroforestry Center: Bogor, Indonesia, 2002. [Google Scholar]



| Items | PN | AS | SA | MA | TD | Note |
|---|---|---|---|---|---|---|
| ||||||
| PM | AM, SM | AA, SM | ML, AA | DL, TG | |
| 8 | 6 | 15 | 10 | 10 | |
| 0.48 a | 0.54 b | 1.11 b | 1.07 b | 0.75 b | ** |
| 27.61 a | 17.56 a | 15.94 a | 19.91 a | 23.55 a | * |
| 375 a | 204 b | 232 b | 15 b | 211 b | * |
| 28.14 a | 19.29 b | 21.29 ab | 21 ab | 27.14 a | * |
| 68.43 a | 75.5 abc | 74.29 abc | 59.71 ab | 85.43 c | ** |
| ||||||
| 244 a | 353 a | 303 a | 319 a | 307 a | ** |
| 28 a | 31 a | 32 a | 32 a | 34 a | ** |
| 18 a | 22 a | 16 a | 19 a | 17 | ** |
| ||||||
| 37.00 a | 30.04 bc | 36.65 ab | 31.35 c | 35.76 ab | ** |
| 25.52 a | 31.26 a | 22.67 a | 29.76 a | 24.77 a | ** |
| 37.5 a | 38.7 a | 40.67 a | 38.89 a | 39.46 a | ** |
| 1.14 a | 1.15 a | 1.19 a | 1.15 a | 1.18 a | * |
| 6.11 a | 6.15 a | 6.17 a | 6.31 a | 6.38 a | * |
| 1.05 a | 1.33 ab | 1.17 ab | 0.99 a | 1.72 b | * |
| 0.20 a | 0.2 ab | 0.26 b | 0.22 ab | 0.24 ab | * |
| ||||||
| 40 a | 54.29 a | 39.29 a | 45 a | 25.71 b | ** |
| 122 a | 143.57 a | 200.71 a | 236.29 a | 255.71 a | * |
| 125.71 a | 331.57 b | 347.29 b | 384.43 b | 344.14 b | ** |
| No | Comparison of the Sorensen Distance | T | A | p-Value |
|---|---|---|---|---|
| 1 | General Comparison | −7.05 | 0.19 | 0.000 |
| 2 | Pairwise Comparison: | |||
| PN vs. AS | −1.65 | 0.05 | 0.066 | |
| PN vs. SA | −5.73 | 0.16 | 0.000 | |
| PN vs. MA | −3.34 | 0.08 | 0.006 | |
| PN vs. TD | −5.97 | 0.32 | 0.001 | |
| AS vs. SA | −1.47 | 0.04 | 0.085 | |
| AS vs. MA | 0.44 | 0.01 | 0.576 | |
| AS vs. TD | −4.81 | 0.22 | 0.002 | |
| SA vs. MA | −0.89 | 0.02 | 0.162 | |
| SA vs. TD | −6.13 | 0.29 | 0.001 | |
| MA vs. TD | −3.49 | 0.17 | 0.012 |
| Stand Type | Species | Ival | p Value | Typical |
|---|---|---|---|---|
| PN | Slaginella sp. Spilanthes paniculata | 48.7 71.4 | 0.005 0.000 | Semi-shade-tolerant Shade-intolerant |
| AS | Swietenia macrophylla | 46.5 | 0.008 | Shade-tolerant |
| SA | Cynodon dactylon | 54.6 | 0.003 | Shade-intolerant |
| MA | Ageratum conyzoides Lantana camara Scleira sp. | 43.6 31.4 27.1 | 0.020 0.024 0.000 | Shade-intolerant Shade-intolerant Shade-intolerant |
| TD | Ottochloa nodosa Andrographis paniculata Bauhinia sp. Nephrolepis sp. Dalbergia latifolia Gmelina elliptica | 60.2 34.4 71.4 42.9 46.5 71.4 | 0.001 0.006 0.000 0.031 0.045 0.000 | Shade-tolerant Semi-shade-tolerant Semi-shade-tolerant Semi-shade-tolerant Shade-tolerant Semi-shade-tolerant |
| Axis 1 | Axis 2 | Axis 3 | |
|---|---|---|---|
| Summary statistic: | |||
| Eigenvalues | 0.436 | 0.336 | 0.281 |
| Variance Explained (%) | 10.200 | 7.800 | 6.500 |
| Cumulative explained (%) | 10.200 | 18.000 | 24.500 |
| Pearson correlation | 0.925 | 0.900 | 0.929 |
| Variables | Veg-H’ | Veg-BA | Veg-Dy | Veg-CH | Veg-CC | Top-Alt | Top-Slope | Ed-Silt | Ed-Sand | Ed-SOC | Ed-TN | Ant-BsU | Ant-River |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veg-H’ | −0.29 ns | −0.147 ns | −0.290 ns | 0.100 ns | 0.210 ns | 0.388 * | −0.002 ns | 0.025 ns | 0.125 ns | 0.480 ** | −0.184 ns | 0.434 ** | |
| Veg-BA | - | 0.602 ** | 0.243 ns | 0.382 * | 0.092 ns | −0.189 ns | 0.171 ns | −0.380 * | 0.131 ns | 0.087 ns | −232 ns | 0.300 ns | |
| Veg-Dy | - | - | 0.329 ns | 0.265 ns | −0.195 ns | −420 * | 0.511 ** | −0.305 ns | 0.098 ns | −0.053 ns | −0.287 ns | −0.292 ns | |
| Veg-CH | - | - | - | 0.125 ns | −0.366 * | −0.308 ns | 0.261 ns | −0.220 ns | 0.088 ns | −246 ns | −561 ** | −0.268 ns | |
| Veg-CC | - | - | - | - | 0.242 ns | −0.172 ns | 0.362 * | −0.259 ns | 0.686 ** | 0.269 ns | −436 * | 0.149 ns | |
| Top-Alt | - | - | - | - | - | 0.479 ** | −0.142 ns | −0.102 ns | −0.034 ns | 0.111 ns | 0.065 ns | 0.614 ** | |
| Top-slope | - | - | - | - | - | - | −0.339 * | −0.033 ns | −0.198 ns | 0.130 ns | 0.199 ns | 0.524 ** | |
| Ed-Silt | - | - | - | - | - | - | - | −0.033 v | 0.311 ns | 0.120 ns | −0.330 ns | −0.264 ns | |
| Ed-sand | - | - | - | - | - | - | - | - | −0.065 ns | 0.140 ns | 0.282 ns | −0.210 ns | |
| Ed-SOC | - | - | - | - | - | - | - | - | - | 0.233 ns | −0.327 ns | −0.22 ns | |
| Ed-TN | - | - | - | - | - | - | - | - | - | - | −0.141 ns | 0.004 ns | |
| Ant-BsU | - | - | - | - | - | - | - | - | - | - | - | −0.070 ns | |
| Ant-River | - | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siswo; Yun, C.-W.; Lee, J. Role of Tree Vegetation and Associated Environmental Factors on the Understory Herb-Layer Composition in a Reforested Area: A Study from “Kulon Progo Community Forestry”. Diversity 2023, 15, 900. https://doi.org/10.3390/d15080900
Siswo, Yun C-W, Lee J. Role of Tree Vegetation and Associated Environmental Factors on the Understory Herb-Layer Composition in a Reforested Area: A Study from “Kulon Progo Community Forestry”. Diversity. 2023; 15(8):900. https://doi.org/10.3390/d15080900
Chicago/Turabian StyleSiswo, Chung-Weon Yun, and Jeongeun Lee. 2023. "Role of Tree Vegetation and Associated Environmental Factors on the Understory Herb-Layer Composition in a Reforested Area: A Study from “Kulon Progo Community Forestry”" Diversity 15, no. 8: 900. https://doi.org/10.3390/d15080900
APA StyleSiswo, Yun, C. -W., & Lee, J. (2023). Role of Tree Vegetation and Associated Environmental Factors on the Understory Herb-Layer Composition in a Reforested Area: A Study from “Kulon Progo Community Forestry”. Diversity, 15(8), 900. https://doi.org/10.3390/d15080900