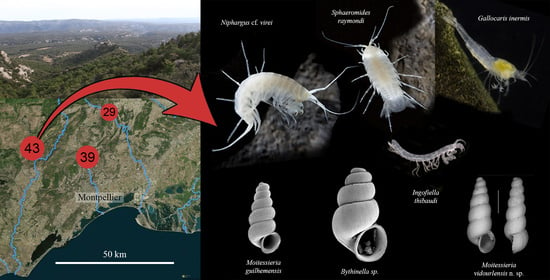
The Cent Fonts Aquifer: An Overlooked Subterranean Biodiversity Hotspot in a Stygobiont-Rich Region
Abstract
:1. Introduction
1.1. Karst and Caves of the North-Montpellier Region
1.2. Description of the Cent Fonts System
1.3. History of Biological Studies
1.4. Threats
1.5. Objectives
2. Materials and Methods
3. Results
3.1. Stygobionts
3.1.1. Clitellata Michaelsen, 1919; Arhynchobdellida Blanchard, 1894
- Trocheta taunensis Grosser, 2015 (=T. bykowskii)
3.1.2. Gastropoda Cuvier, 1795; Littorinimorpha Golikov & Starobogatov, 1975
Amnicolidae Tryon, 1863
- Bythinella sp.
Hydrobiidae Stimpson, 1865
- Heraultiella exilis (Paladilhe, 1867)
- Islamia cf. moquiniana (Dupuy, 1851)
Moitessieriidae Bourguignat, 1864
- Paladilhia pleurotoma Bourguignat, 1865
- Bythiospeum bourguignati (Paladilhe, 1866)
- Moitessieria vidourlensis n. sp. (=Moitessieria rolandiana Bourguignat, 1864)
- Moitessieria guilhemensis Girardi & Boeters, 2017
- Moitessieria n. sp.?
3.1.3. Malacostraca Latreille, 1802
Decapoda Latreille, 1802
- Gallocaris (Troglocaris) inermis (Fage, 1937)
Isopoda Latreille, 1816
- Stenasellus buili Rémy, 1949
- Proasellus cavaticus (Leydig, 1871)
- Microcharon doueti Coineau, 1968
- Faucheria faucheri (Dollfus & Viré, 1900)
- Sphaeromides raymondi Dollfus, 1897
Amphipoda Latreille, 1816
- Niphargus laisi Schellenberg, 1936
- Niphargus gallicus Schellenberg, 1935
- Niphargus kochianus Bate, 1859
- Niphargus pachypus Schellenberg, 1933
- Niphargus cf. virei Chevreux, 1896 Clade A
- Salentinella angelieri Delamare-Deboutteville & Ruffo, 1952
- Salentinella delamarei Coineau, 1962
Ingolfiellida Hansen, 1903
- Ingolfiella thibaudi Coineau, 1968
Bathynellacea Chappuis, 1915
- Gallobathynella (Clamousella) delayi Serban, Coineau & Delamare Deboutteville 1971
3.1.4. Ostracoda Latreille, 1802; Podocopida Sars, 1866
- Fabaeformiscandona cf. breuili (Paris, 1920)
- Marmocandona cf. zschokkei (Wolf, 1920)
- Schellencandona cf. simililampadis (Danielpol, 1978)
- Sphaeromicola cebennica juberthiei Danielpol, 1977
- Candoninae sp. 1, 2, 3
3.1.5. Copepoda Milne Edwards, 1840
Cyclopoida Burmeister, 1834
- Acanthocyclops rhenanus Kiefer, 1936
- Acanthocyclops venustus (stammeri) cf. westfalicus (Kiefer, 1931)
- Graeteriella boui Lescher-Moutoué, 1974
- Graeteriella unisetigera (Graeter, 1908)
- Graeteriella (Paragraeteriella) vandeli Lescher-Moutoué, 1969
- Kieferiella delamarei (Lescher-Moutoué, 1971)
- Speocyclops racovitzai (Chappuis, 1923)
Harpacticoida Sars G.O., 1903
- Ceuthonectes gallicus Chappuis, 1928
- Elaphoidella leruthi meridionalis Chappuis, 1953
- Nitocrella omega Hertzog, 1936
- Nitocrella hirta Chappuis, 1924
- Pseudectinosoma vandeli (Rouch, 1969)
3.1.6. Arachnida, Acari
- Soldanellonyx chappuisi Walter, 1917
3.2. Troglobionts
3.2.1. Araneae
- Palliduphantes sanctivincenti (Simon, 1873)
3.2.2. Opiliones
- Peltonychia clavigera (Simon, 1872)
3.2.3. Pseudoscorpiones
- Neobisium tuzetae Vachon, 1947
3.2.4. Isopoda
- Trichoniscoides bonneti Vandel, 1946
3.2.5. Diplura
- Plusiocampa balsani Conde, 1947
3.2.6. Collembola
- Pseudosinella denisi Gisin, 1954
- Onychiurus ortus Denis, 1935
3.2.7. Coleoptera
- Laemostenus (Actenipus) oblongus balmae (Delarouzée, 1860)
3.3. Stygophilic taxa
- Eucyclops serrulatus (Fisher, 1851);
- Paracyclops fimbriatus (Fisher, 1853);
- Acanthocyclops vernalis (Fisher, 1853);
- Megacyclops viridis (Jurine, 1820), in the hyporheic zone of the Hérault River near the Cent Fonts exurgences;
- Diacyclops languidoides Lilljeborg, 1901.
3.4. Troglophilic taxa and Parasites
- Ixodida
- Eschatocephalus vespertilionis (Koch, 1844) is a common bat parasite.
- Araneae
- Lessertia dentichelis (Simon, 1884), a troglophile species, very common in the caves throughout the Hérault valley.
- Meta bourneti Simon, 1922, troglophile, very common in all caves in the area.
- Meta menardi (Latreille, 1804), troglophile, very common in all caves in the area.
- Opiliones
- Sabacon paradoxus Simon, 1879, troglophile, found in cave entrances in France and Spain. It is very common in most caves in the Cévennes and in the Hérault karsts.
- Julida
- Blaniulus guttulatus (Fabricius, 1798), troglophile, common in all caves of the region.
- Isopoda
- Oritoniscus delmasi delmasi Vandel, 1933, endogeous and troglophile species, endemic to the southern Cévennes between the Vidourle and Lergue Rivers.
- Phymatoniscus propinquus (Brian, 1908), troglophile. The ocular area of this species is generally provided with a large single eyespot but in specimens of the Cents Fonts cave, eyes are completely invisible to external examination [43]. The species is common throughout the Cévennes in the Ardèche, Gard, and Hérault departments.
- Coleoptera
- Leptinus testaceus P.W. Müller, 1817, is a troglophile, ectoparasite, and commensal of many species of micromammals. It lives mainly in subterranean mammal nests as well as in caves, on bat guano. This species is sporadic but known from many caves around the Cent Fonts system.
4. Discussion
4.1. A Biodiversity Hotspot Embedded in a Stygobiont Species-Rich Area
4.2. Conservation Issues and Threats
4.3. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Material and Methods
Appendix A.1.1. Biogeography
Appendix A.1.2. Morphometrics

Appendix A.1.3. DNA Analyses
Appendix A.2. Results
Appendix A.2.1. Biogeography

Appendix A.2.2. Morphometry

Appendix A.2.3. Genetics
| Specimen | Location | COI | 16S | 28S |
|---|---|---|---|---|
| Moitessieria rolandiana | 4 1 | PP050555 | ||
| Moitessieria rolandiana | 4 1 | PP050556 | PP051255 | |
| Moitessieria larzacensis n. sp. | 1 | PP050554 | PP051254 | |
| Moitessieria larzacensis n. sp. | 6 | PP050557 | PP051256 | |
| Moitessieria atlantica n. sp. 1320 | 5 | PP057732 | ||
| Moitessieria atlantica n. sp. 1321 | 5 | PP051257 | PP057733 | |
| Moitessieria atlantica n. sp. | 5 | PP057734 | ||
| Moitessieria atlantica n. sp. | 5 | PP057735 | ||
| Moitessieria vidourlensis n. sp. | 3 | PP057731 | ||
| Moitessieria vidourlensis n. sp. | 2 | PP057736 | ||
| Moitessieria vidourlensis n. sp. | 2 | PP057737 |
- (i)
- M. larzacensis n. sp. differs from M. rolandiana based on both COI and 16S.
- (ii)
- M. atlantica n. sp. differs from M. rolandiana and M. larzacensis n. sp. based on 16S.
- (iii)
- M. vidourlensis n. sp. differs from M. atlantica n. sp. based on 28S, but cannot be compared to M. rolandiana nor M. larzacensis n. sp. from the available molecular data. Only morphological differences, that are supported by geographical isolation, allow this species to be separated from M. rolandiana and M. larzacensis n. sp.

Appendix A.2.4. Species Delimitation
Appendix A.2.5. Species Turbo-Taxonomy



References
- Malard, F.; Gibert, J.; Laurent, R. L’aquifère de la source du Lez: Un réservoir d’eau… et de biodiversité. Karstologia 1997, 30, 49–54. [Google Scholar] [CrossRef]
- Culver, D.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Tuzet, O.; Bonnet, A.; Bournier, E.; Du Cailar, J. Troisième contribution à la faune du Languedoc méditerranéen. Notes Biospéologiques 1950, 5, 85–95. [Google Scholar]
- Balazuc, J.; Bonnet, A.; Bournier, E.; Du Cailar, J. Crustacés des eaux souterraines du Languedoc. Remarques sur leur répartition? Bull. Soc. Hist. Nat. Toulouse 1951, 86, 80–87. [Google Scholar]
- Bonnet, A.; Bournier, E.; Du Cailar, J.; Quezel, P. Sur quatre crustacés aquatiques et troglobies d’une résurgence des gorges de l’Héralut. Soc. Mér. Spéléologie Préhistoire 1951, 86, 341–346. [Google Scholar]
- Rouch, R.; Juberthie-Jupeau, L.; Juberthie, C. Recherche sur les eaux souterraines—3—Essai d’étude du peuplement de la zone noyée d’un karst. Ann. Spéléol. 1968, 23, 717–733. [Google Scholar]
- Olivier, M.-J.; Martin, D.; Bou, C.; Prié, V. Interprétation du Suivi Hydrobiologique de la Faune Stygobie, Réalisé sur le Système Karstique des Cent Fonts Lors du Pompage D’essai; BRGM/RP+54865-FR; Conseil Général de l’Hérault: Montpellier, France, 2006; p. 42. [Google Scholar]
- Prié, V. Systématique et Micro-Répartition des Mollusques Stygobies des Karsts du Nord-Montpelliérain. Master’s Thesis, De l’École Pratique des Hautes Études, Paris, France, 2006. [Google Scholar]
- Prié, V. Taxonomie et Biogéographie des Mollusques Patrimoniaux: Quelles échelles Pour la Délimitation des Taxons et des unités de Gestion? Ph.D. Thesis, Muséum National d’Histoire Naturelle, Paris, France, 2013. [Google Scholar]
- Girardi, H. Moitessieria wienini n. sp. des eaux de l’Aquifère de la Montagne de la Sellette, sur la rivière Hérault, (F. 34), (Mollusca: Gastéropoda: Moitessieriidae). Doc. Malacol. 2001, 2, 3–10. [Google Scholar]
- Girardi, H. Contribution à l’étude des gastéropodes stygobies de France. 3—Paladilhia conica (PALADILHE, 1867) (Gastropoda: Moitessieriidae). Doc. Malacol. 2003, 4, 89–90. [Google Scholar]
- Ladouche, B.; Maréchal, J.C.; Dörfliger, N.; Lachassagne, P.; Lanini, S.; Le Strat, P. Pompages d’Essai sur le Système Karstique des Cent Fonts (Cne de Causse de la Selle, Hérault), Présentation et Interprétation des Données Recueillies, BRGM/RP 54426-FR, 2005. Available online: https://infoterre.brgm.fr/rapports/RP-54426-FR.pdf (accessed on 24 November 2023).
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Bou, C.; Rouch, R. Un nouveau champ de recherches sur la faune aquatique souterraine. C. R. Acad. Sci. 1967, 265, 369–370. [Google Scholar]
- Jourde, H.; Dörfliger, N.; Maréchal, J.C.; Batiot-Guilhe, C.; Bouvier, C.; Courrioux, G.; Desprats, J.-F.; Fullgraf, T.; Ladouche, B.; Leonardi, V.; et al. Projet Gestion Multi-Usages de L’hydrosystème Karstique du Lez-Synthèse des Connaissances Récentes et Passées. BRGM/RP-60041-FR, 2011. Available online: https://brgm.hal.science/hal-03143770/document (accessed on 24 November 2023).
- Bertrand, A. Atlas préliminaire de répartition géographique des mollusques stygobies de la faune de France (MOLLUSCA: RISSOIDEA: CAENOGASTROPODA). Doc. Malacol. 2004, 2, 82. [Google Scholar] [CrossRef]
- UICN Comite Français; OFB; MNHN. La Liste Rouge des Espèces Menacées en France—Chapitre Mollusques Continentaux de France Métropolitaine; UICN: Paris, France, 2021. [Google Scholar]
- UICN France; MNHN. La Liste Rouge des Espèces Menacées en France—Chapitre Crustacés d’eau Douce de France Métropolitaine; UICN: Paris, France, 2012. [Google Scholar]
- Lecaplain, B. Sur la présence en France de Trocheta taunensis Grosser, 2015 (Hirudinida, Erpobdellidae). Naturae 2021, 25, 345–349. [Google Scholar] [CrossRef]
- Sket, B. K Poznavanju Favne Pijavk (Hirudinea) v Jugoslaviji, Zur Kenntnis der Egel-Fauna (Hirudinea) Jugoslawiens. Acad. Sci. Artium Slov. Cl. IV Hist. Nat. Med. 1968, 9, 127–197. [Google Scholar]
- Grosser, C. Differentiation of some similar species of the subfamily Trochetinae (Hirudinida: Erpobdellidae). Ecol. Montenegrina 2015, 2, 29–41. [Google Scholar] [CrossRef]
- Prié, V. Répartition de Heraultiella exilis (Paladilhe, 1867) (Gastropoda, Caenogastropoda, Rissooidea). MalaCo 2005, 1, 8–9. [Google Scholar]
- Prié, V. Heraultiella exilis. The IUCN Red List of Threatened Species 2011: e.T2092A9236035. 2011. Available online: https://www.iucnredlist.org/species/2092/9236035 (accessed on 24 November 2023).
- Prié, V. Paladilhia pleurotoma. The IUCN Red List of Threatened Species 2010: e.T15876A5275513. 2010. Available online: https://www.iucnredlist.org/species/15876/5275513 (accessed on 24 November 2023).
- Prié, V. Bythiospeum bourguignati. The IUCN Red List of Threatened Species 2010: e.T61315A12461687. 2010. Available online: https://www.iucnredlist.org/species/61315/12461687 (accessed on 24 November 2023).
- Callot-Girardi, H.; Boeters, H.D. Moitessieria guilhemensis, nouvelle espèce de la résurgence du Cabrier à Saint-Guilhem-le Désert, Hérault, France. (Mollusca: Caenogastropoda: Moitessieriidae). Avenionia 2017, 2, 42–63. [Google Scholar]
- De Grave, S. Gallocaris inermis. The IUCN Red List of Threatened Species 2013: e.T198319A2520643. Available online: https://www.iucnredlist.org/species/198319/2520643 (accessed on 1 December 2023).
- Henry, J.-P. Contribution à l’étude du genre Proasellus (crustacea isopoda asellidae): Le groupe cavaticus. Vie Milieu 1971, 22, 33–77. [Google Scholar]
- Bertrand, J.-Y. Recherches Sur l’Écologie de Faucheria faucheri (Crustacés, Cirolanides). Ph.D. Thesis, Université de Paris VI, Paris, France, 1974; p. 123. [Google Scholar]
- Fiser, C.; Zagmajster, M.; Dethier, M. Overview of Niphargidae (Crustacea: Amphipoda) in Belgium: Distribution, taxonomic notes and conservation issues. Zootaxa 2018, 4387, 26. [Google Scholar] [CrossRef] [PubMed]
- McInerney, C.E.; Maurice, L.; Robertson, A.L.; Knight, L.R.; Arnscheidt, J.; Venditti, C.; Dooley, J.; Mathers, T.C.; Matthijs, S.; Rrikkson, K.; et al. The ancient Britons: Groundwater fauna survived extreme climate change over tens of millions of years across NW Europe. Mol. Ecol. 2014, 23, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.H.; Gledhill, T. The Niphargus kochianus—group in North-Western Europe. Crustac. Suppl. 1977, 4, 212–243. [Google Scholar]
- Lefébure, T.; Douady, C.J.; Gouy, M.; Trontelj, P.; Briolay, J.; Gibert, J. Phylogeography of a subterranean amphipod reveals cryptic diversity and dynamic evolution in extreme environments. Mol. Ecol. 2006, 15, 1797–1806. [Google Scholar] [CrossRef]
- Danielpol, D.L.; Namiotko, T.; Meisch, C. Marmocandona nov. gen. (Ostracoda, Candoninae), with comments on the contribution of stygobitic organisms to micropalaeontological studies. Kölner Forum Geol. Paläont. 2012, 21, 13–16. [Google Scholar]
- Walter, T.C.; Boxshall, G. World of Copepods Database. Acanthocyclops venustus venustus (Norman & Scott T., 1906). 2023, Accessed through: World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=729873on2023-11-08 (accessed on 11 November 2023).
- Lescher-Moutoué, F. Recherches sur les eaux souterraines—21—Un Cyclopide nouveau du genre Graeteriella. Ann. Spéléol. 1974, 29, 71–76. [Google Scholar]
- Fiers, F.; Ghenne, V. Cryptozoic copepods from Belgium: Diversity and biogeographic implications. Belg. J. Zool. 2000, 130, 11–19. [Google Scholar]
- Lescher-Moutoué, F. Recherches sur les eaux souterraines—7—Les cyclopides de la zone noyée d’un karst. I Graeteriella (Paragraeteriella) vandeli n. sp. Ann. Spéléol. 1969, 24, 429–438. [Google Scholar]
- Lescher-Moutoué, F. Sur la biologie et l’écologie des Copépodes Cyclopides hypogés. Ann. Spéléol. 1973, 28, 429–502 & 581–674. [Google Scholar]
- Iannella, M.; Fiasca, B.; Di Lorenzo, T.; Biondi, M.; Di Cicco, M.; Galassi, D.M.P. Spatial distribution of stygobitic crustacean harpacticoids at the boundaries of groundwater habitat types in Europe. Sci. Rep. 2020, 10, 19043. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; Huys, R.; Reid, J.W. Diversity, ecology and evolution of groundwater copepods. Freshw. Biol. 2009, 54, 691–708. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; De Laurentiis, P.; Dole-Olivier, M.-J. Phylogeny and biogeography of the genus Pseudectinosoma, and description of P. janineae sp. n. (Crustacea, Copepoda, Ectinosomatidae). Zool. Scr. 1999, 28, 289–303. [Google Scholar] [CrossRef]
- Vandel, A. Isopodes Terrestres (Première Partie); Office Central de Faunistique, Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1960; p. 417. [Google Scholar]
- Juberthie, C.; Juberthie-Jupeau, L. La réserve biologique du laboratoire souterrain du C.N.R.S. à Sauve (Gard). Ann. Spéléol. 1975, 30, 539–555. [Google Scholar]
- Delamare Debouteville, C. Biologie des Eaux Souterraines Littorales et Continentales; Hermann: Paris, France, 1960; 740p. [Google Scholar]
- Wilke, T.; Davis, G.; Falniowski, A.; Giusti, F.; Bodon, M.; Szarowska, M. Molecular systematics of Hydrobiidae (Mollusca: Gastropoda: Rissooidea): Testing monophyly and phylogenetic relationships. Proc. Acad. Nat. Sci. USA 2009, 151, 1–21. [Google Scholar] [CrossRef]
- Machetel, P.; Yuen, D.A. Revisiting Cent-Fonts Fluviokarst Hydrological Properties with Conservative Temperature Approximation. Hydrology 2017, 4, 6. [Google Scholar] [CrossRef]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F.; et al. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef]
- Prié, V.; Danet, A.; Valentini, A.; Lopes-Lima, M.; Taberlet, P.; Besnard, A.; Roset, N.; Gargominy, O.; Dejean, T. Conservation assessment based on large-scale monitoring of eDNA: Application to freshwater mussels. Biol. Cons. 2023, 283, 110089. [Google Scholar] [CrossRef]
- Fukuda, H.; Haga, T.; Tatara, Y. Niku-nuki: A useful method for anatomical and DNA studies on shell-bearing molluscs. Zoosymposia 2008, 1, 15–38. [Google Scholar] [CrossRef]
- SANDRE. Base de Données Sur l’Hydrographie. 2007. Available online: http://sandre.eaufrance.fr/ (accessed on 14 November 2023).
- Wilcox, D.; Dove, B.; McDavid, D.; Greer, D. Image Tool for Windows version 3.00. 2002. UTHSCA ed. San Antonio: UTHSCA. Available online: http://ddsdx.uthscsa.edu/dig/itdesc.html (accessed on 3 July 2005).
- Bernasconi, R. Révision du genre Bythinella (Moquin-Tandon, 1855) de la France du Centre-Ouest, du Midi et des Pyrénées (Gastropoda Prosobranchia Hydrobiidae: Amnicolinae Bythinellini). Doc. Malacol. 2000, 1, 126. [Google Scholar]
- Haase, M.; Gargominy, O.; Fontaine, B. Rissoidean freshwater gastropods from the middle of the Pacific: The genus Fluviopupa on the Austral Islands (Caenogastropoda). Molluscan Res. 2005, 25, 145–163. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 3 May 2013).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Palumbi, S.R. Nucleic acids II: The Polymerase Chain Reaction. In Molecular Systematics, 2nd ed.; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer: Sunderland, MA, USA, 1996; pp. 205–247. [Google Scholar]
- Jovelin, R.; Justine, J.-L. Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. Int. J. Parasitol. 2001, 31, 393–401. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Hershler, R.; Liu, H.P.; Thompson, F.G. Phylogenetic relationships of North American nymphophiline gastropods based on mitochondrial DNA sequences. Zool. Scr. 2003, 32, 357–366. [Google Scholar] [CrossRef]
- Hurt, C.R. Genetic divergence, population structure and historical demography of rare spring-snails (Pyrgulopsis) in the lower Colorado River basin. Mol. Ecol. 2004, 13, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Hershler, R.; Clift, K. Mitochondrial DNA sequences reveal extensive cryptic diversity within a western American springsnail. Mol. Ecol. 2003, 12, 2771–2782. [Google Scholar] [CrossRef]
- Prié, V.; Bichain, J.-M. Phylogenetic relationships and description of a new stygobite species of Bythinella (Mollusca, Gastropoda, Caenogastropoda, Amnicolidae) from southern France. Zoosystema 2009, 31, 987–1000. [Google Scholar] [CrossRef]








| Classe | Sous-Classe | Ordre | [3,4,5] | Rouch et al. [6] | Olivier et al. [7] | This Paper |
|---|---|---|---|---|---|---|
| Clitellata | Hirudinea | Arhynchobdellida | - | Trocheta taunensis Grosser, 2015 | ||
| Gastropoda | Caenogastropoda | Littorinimorpha | - | - | Bythinella n. sp. | Bythinella sp. |
| - | - | Heraultiella exilis | Heraultiella exilis (Paladilhe, 1867) | |||
| - | - | lslamia moquiniana | lslamia cf. moquiniana | |||
| - | - | Paladilhia pleurotoma | Paladilhia pleurotoma Bourguignat, 1865 | |||
| - | - | Bythiospeum bourguignati | Bythiospeum bourguignati (Paladilhe, 1866) | |||
| - | - | Moitessieria rolandiana | Moitessieria vidourlensis n. sp. | |||
| - | - | Moitessieria n. sp. 1 | Moitessieria guilhemensis Girardi & Boeters, 2017 | |||
| - | - | Moitessieria n. sp. 2? | Moitessieria sp. | |||
| Malacostraca | Eumalacostraca | Decapoda | Troglocaris inermis | Troglocaris inermis | Troglocaris inermis | Gallocaris (Troglocaris) inermis (Fage, 1937) |
| Isopoda | - | Stenasellus buili | Stenasellus buili | Stenasellus buili Rémy, 1949 | ||
| - | Proasellus cavaticus cavaticus | Proasellus cavaticus | Proasellus cavaticus (Leydig, 1871) | |||
| - | Microcharon doueti n. sp. | Microcharon doueti | Microcharon doueti Coineau, 1968 | |||
| Faucheria faucheri | Faucheria faucheri | Faucheria faucheri | Faucheria faucheri (Dollfus & Viré, 1900) | |||
| Sphaeromides raymondi | Sphaeromides raymondi | - | Sphaeromides raymondi Dollfus, 1897 | |||
| Amphipoda | - | - | Niphargus laisi | Niphargus laisi Schellenberg, 1936 | ||
| - | - | Niphargus gallicus | Niphargus gallicus Schellenberg, 1935 | |||
| - | - | Niphargus kochianus | Niphargus kochianus Bate, 1859 | |||
| - | - | Niphargus pachypus | Niphargus pachypus Schellenberg, 1933 | |||
| Niphargus orcinus virei | Niphargus orcinus virei | Niphargus virei | Niphargus cf. virei Chevreux, 1896 clade A | |||
| - | Salentinella sp. | Salentinella angelieri | Salentinella angelieri Delamare-Deboutteville & Ruffo, 1952 | |||
| - | Salentinella delamarei | Salentinella delamarei Coineau, 1962 | ||||
| Ingolfiellida | - | Ingofiella sp. | Ingolfiella thibaudi | Ingolfiella thibaudi Coineau, 1968 | ||
| Bathynellacea | - | - | Clamousella cf. delayi | Gallobathynella delayi Serban, Coineau & Delamare Deboutteville | ||
| Ostracoda | Podocopa | Podocopida | - | - | Fabaeformiscandona cf. breuili | Fabaeformiscandona cf. breuili (Paris, 1920) |
| - | - | Pseudocandona zschokkei | Marmocandona cf. zschokkei (Wolf, 1920) | |||
| - | - | Schellencandona cf. simililampadis | Schellencandona cf. simililampadis (Danielpol, 1978) | |||
| Sphaeromicola cebennica | Sphaeromicola cebennica | - | Sphaeromicola cebennica juberthiei Danielpol, 1977 | |||
| - | - | Candoninae sp. 1, long, related to Cryptocandona | Candoninae sp. 1, cf. Cryptocandona | |||
| - | - | Candoninae sp. 2, bean-shaped, related to Pseudocandona | Candoninae sp. 2, cf. Pseudocandona | |||
| - | - | Candoninae sp. 3, triangular, related to Pseudocandona group eremita | Candoninae sp. 3, cf. Pseudocandona group eremita | |||
| Copepoda | Neocopepoda | Cyclopoida | - | Acanthocyclops rhenanus | - | Acanthocyclops rhenanus Kiefer, 1936 |
| - | Acanthocyclops stammeri westfalicus | Acanthocyclops venustus westfalicus | Acanthocyclops venustus westfalicus (Kiefer, 1931) | |||
| - | - | Graeteriella (Graeteriella) cf. boui | Graeteriella (Graeteriella) boui Lescher-Moutoué, 1969 | |||
| - | Graeteriella unisetiger | - | Graeteriella (Graeteriella) unisetigera Graeter, 1910 | |||
| - | Paragraeteriella n. sp. | - | Graeteriella (Paragraeteriella) vandeli Lescher-Moutoué, 1969 | |||
| - | - | Kieferiella delamarei | Kieferiella delamarei (Lescher-Moutoué, 1971) | |||
| - | Speleocyclops sp. | - | Speocyclops racovitzai Chappuis, 1923 | |||
| Harpacticoida | - | Ceuthonectes gallicus | Ceuthonectes gallicus | Ceuthonectes gallicus Chappuis, 1928 | ||
| - | Elaphoidella leruthi meridionalis | Elaphoidella leruthi meridionalis | Elaphoidella leruthi meridionalis Chappuis, 1953 | |||
| - | - | Nitocrella omega | Nitocrella omega Hertzog, 1936 | |||
| - | Nitocrella cf. hirta | Nitocrella hirta hirta | Nitocrella hirta Chappuis, 1924 | |||
| - | Ectinosomidae sp. | Pseudectinosoma vandeli | Pseudectinosoma vandeli (Rouch, 1969) | |||
| Arachnida | Acari | Trombidiformes | - | Soldanellonyx chappuisi | - | Soldanellonyx chappuisi Walter, 1917 |
| Insecta | Pterygota | Coleoptera | Laemostenus (Actenipus) oblongus balmae (Delarouzée, 1860) 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prié, V.; Alonso, C.; Bou, C.; Galassi, D.M.P.; Marmonier, P.; Dole-Olivier, M.-J. The Cent Fonts Aquifer: An Overlooked Subterranean Biodiversity Hotspot in a Stygobiont-Rich Region. Diversity 2024, 16, 50. https://doi.org/10.3390/d16010050
Prié V, Alonso C, Bou C, Galassi DMP, Marmonier P, Dole-Olivier M-J. The Cent Fonts Aquifer: An Overlooked Subterranean Biodiversity Hotspot in a Stygobiont-Rich Region. Diversity. 2024; 16(1):50. https://doi.org/10.3390/d16010050
Chicago/Turabian StylePrié, Vincent, Cédric Alonso, Claude Bou, Diana Maria Paola Galassi, Pierre Marmonier, and Marie-José Dole-Olivier. 2024. "The Cent Fonts Aquifer: An Overlooked Subterranean Biodiversity Hotspot in a Stygobiont-Rich Region" Diversity 16, no. 1: 50. https://doi.org/10.3390/d16010050
APA StylePrié, V., Alonso, C., Bou, C., Galassi, D. M. P., Marmonier, P., & Dole-Olivier, M. -J. (2024). The Cent Fonts Aquifer: An Overlooked Subterranean Biodiversity Hotspot in a Stygobiont-Rich Region. Diversity, 16(1), 50. https://doi.org/10.3390/d16010050