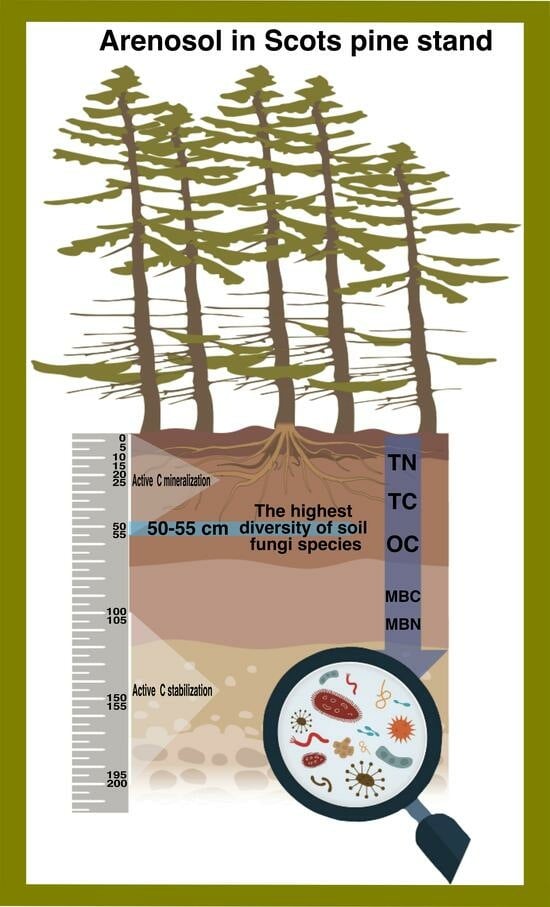
Soil Fungi and Soil Organic Carbon Stocks in the Profile of a Forest Arenosol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.1.1. Study Site
2.1.2. Sampling
2.2. Soil Chemical Parameters
2.3. Soil Microbial Biomass Carbon and Nitrogen Estimation
2.4. DNA Extraction, Amplification, and Sequencing
2.5. Bioinformatics
2.6. Statistical Analysis
3. Results
3.1. Soil Chemical Parameters
3.2. Soil Microbial Biomass Carbon and Nitrogen
3.3. Abundance and Diversity of Soil Fungi
3.4. Fungal Guilds
3.5. Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Smal, H.; Ligęza, S.; Pranagal, J.; Urban, D.; Pietruczyk-Popławska, D. Changes in the stocks of soil organic carbon, total nitrogen and phosphorus following afforestation of post-arable soils: A chronosequence study. For. Ecol. Manag. 2019, 451, 117536. [Google Scholar] [CrossRef]
- Bārdule, A.; Butlers, A.; Lazdiņš, A.; Līcīte, I.; Zvirbulis, U.; Putniņš, R.; Jansons, A.; Adamovičs, A.; Razma, Ģ. Evaluation of soil organic layers thickness and soil organic carbon stock in hemiboreal forests in Latvia. Forests 2021, 12, 840. [Google Scholar] [CrossRef]
- Varnagirytė-Kabašinskienė, I.; Žemaitis, P.; Armolaitis, K.; Stakėnas, V.; Urbaitis, G. Soil organic carbon stocks in afforested agricultural land in Lithuanian hemiboreal forest zone. Forests 2021, 12, 1562. [Google Scholar] [CrossRef]
- Borůvka, L.; Vašát, R.; Šrámek, V.; Hellebrandová, K.N.; Fadrhonsová, V.; Sáňka, M.; Pavlů, L.; Sáňka, O.; Vacek, O.; Němeček, K.; et al. Predictors for digital mapping of forest soil organic carbon stocks in different types of landscape. Soil Water Res. 2022, 17, 69–79. [Google Scholar] [CrossRef]
- Liski, J.; Perruchoud, D.; Karjalainen, T. Increasing carbon stocks in the forest soils of western Europe. For. Ecol. Manag. 2002, 169, 159–175. [Google Scholar] [CrossRef]
- Baritz, R.; Seufert, G.; Montanarella, L.; Van Ranst, E. Carbon concentrations and stocks in forest soils of Europe. For. Ecol. Manag. 2010, 260, 262–277. [Google Scholar] [CrossRef]
- Forsmark, B.; Nordin, A.; Maaroufi, N.I.; Lundmark, T.; Gundale, M.J. Low and high nitrogen deposition rates in northern coniferous forests have different impacts on aboveground litter production, soil respiration, and soil carbon stocks. Ecosystems 2020, 23, 1423–1436. [Google Scholar] [CrossRef]
- Li, M.; Meador, T.; Sauheitl, L.; Guggenberger, G.; Angst, G. Substrate quality effects on stabilized soil carbon reverse with depth. Geoderma 2022, 406, 115511. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W. Soil organic matter in major pedogenic soil groups. Geoderma 2021, 384, 114785. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, W.; Zhou, Y.; Yin, Y. Soil organic carbon chemical functional groups under different revegetation types are coupled with changes in the microbial community composition and the functional genes. Forests 2019, 10, 240. [Google Scholar] [CrossRef]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Chang. Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef] [PubMed]
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of clay minerals on some soil fertility attributes: A review. Open J. Soil Sci. 2019, 9, 155–188. [Google Scholar] [CrossRef]
- Derenne, S.; Tu, T.T.N. Characterizing the molecular structure of organic matter from natural environments: An analytical challenge. C. R. Geosci. 2014, 346, 53–63. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Chai, Y.N.; Lopes, L.D.; Ordóñez, R.A.; Wright, E.E.; Archontoulis, S.; Schachtman, D.P. The effects of soil depth on the structure of microbial communities in agricultural soils in Iowa (United States). Appl. Environ. Microbiol. 2021, 87, e02673-20. [Google Scholar] [CrossRef]
- Blaško, R.; Bach, L.H.; Yarwood, S.A.; Trumbore, S.E.; Högberg, P.; Högberg, M.N. Shifts in soil microbial community structure, nitrogen cycling and the concomitant declining N availability in ageing primary boreal forest ecosystems. Soil Biol. Biochem. 2015, 91, 200–211. [Google Scholar] [CrossRef]
- Chodak, M.; Klimek, B.; Niklińska, M. Composition and activity of soil microbial communities in different types of temperate forests. Biol. Fertil. Soils 2016, 52, 1093–1104. [Google Scholar] [CrossRef]
- Awad, A.; Majcherczyk, A.; Schall, P.; Schröter, K.; Schöning, I.; Schrumpf, M.; Ehbrecht, M.; Boch, S.; Kahl, T.; Bauhus, J.; et al. Ectomycorrhizal and saprotrophic soil fungal biomass are driven by different factors and vary among broadleaf and coniferous temperate forests. Soil Biol. Biochem. 2019, 131, 9–18. [Google Scholar] [CrossRef]
- Li, J.; Zhu, T.; Singh, B.K.; Pendall, E.; Li, B.; Fang, C.; Nie, M. Key microorganisms mediate soil carbon-climate feedbacks in forest ecosystems. Sci. Bull. 2021, 66, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Bell-Doyon, P.; Bellavance, V.; Bélanger, L.; Mazerolle, M.J.; Villarreal-A., J.C. Bacterial, fungal, and mycorrhizal communities in the soil differ between clearcuts and insect outbreaks in the boreal forest 50 years after disturbance. For. Ecol. Manag. 2022, 523, 120493. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, S.; Fang, H.; Guo, Y.; Li, Y.; Zhou, Y. Interactions between soil organic matter chemical structure and microbial communities determine the spatial variation of soil basal respiration in boreal forests. Appl. Soil Ecol. 2023, 183, 104743. [Google Scholar] [CrossRef]
- Frey, B.; Walthert, L.; Perez-Mon, C.; Stierli, B.; Köchli, R. Deep soil layers of drought-exposed forests harbor poorly known bacterial and fungal communities. Front. Microbiol. 2021, 12, 674160. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef]
- Crowther, C.R.; Boddy, L.; Jones, T.H. Functional and ecological consequences of saprotrophic fungus–grazer interactions. ISME J. 2012, 6, 1992–2001. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Nelson, S.E.; Wilkinson, K.G.; Smith, S.E.; McKenzie, B.M. Stabilisation of soil against wind erosion by six saprotrophic fungi. Soil Biol. Biochem. 2012, 50, 134–141. [Google Scholar] [CrossRef]
- Amorim, H.C.; Hurtarte, L.C.; Souza, I.F.; Zinn, Y.L. C:N ratios of bulk soils and particle-size fractions: Global trends and major drivers. Geoderma 2022, 425, 116026. [Google Scholar] [CrossRef]
- Gorliczay, E.; Boczonádi, I.; Kiss, N.É.; Tóth, F.A.; Pabar, S.A.; Biró, B.; Kovács, L.R.; Tamás, J. Microbiological effectivity evaluation of new poultry farming organic waste recycling. Agriculture 2021, 11, 683. [Google Scholar] [CrossRef]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) IPCC Guidelines for National Greenhouse Gas Inventories. The National Greenhouse Gas Inventories Programme; Institute for Global Environmental Strategies: Hayama, Japan, 2006; ISBN 4-88788-032-4. [Google Scholar]
- EEA. Europe’s Biodiversity—Biogeographical Regions and Seas; EEA Report No. 1/2002; EEA: Geneva, Switzerland, 2002. [Google Scholar]
- Hytteborn, H.; Maslov, A.A.; Nazimova, D.I.; Rysin, L.P. Boreal forests of Eurasia. In Coniferous Forests. Ecosystems of the World; Andersson, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 23–29. [Google Scholar]
- Jögiste, K.; Korjus, H.; Stanturf, J.A.; Frelich, L.E.; Baders, E.; Donis, J.; Jansons, A.; Kangur, A.; Köster, K.; Laarmann, D.; et al. Hemiboreal forest: Natural disturbances and the importance of ecosystem legacies to management. Ecosphere 2017, 8, e01706. [Google Scholar] [CrossRef]
- Krasnova, A.; Soosaar, K.; Uri, V.; Mander, Ü.; Krasnov, D.; Noe, S. Hemiboreal forests under the 2018 Europe heat wave. Geophys. Res. Abstr. 2019, 21, EGU2019-8604. [Google Scholar]
- Lithuanian Hydrometeorological Service under the Ministry of Environment. Comparative Evaluation of Climate Changes in Lithuania 1961–1990 and 1991–2020 Standard Climate Norms; Lithuanian Hydrometeorological Service under the Ministry of Environment: Vilnus, Lithuania, 2021; p. 18. (In Lithuanian) [Google Scholar]
- International Union of Soil Sciences Working Group World Reference Base. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Food and Agriculture Organization (FAO). Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006. [Google Scholar]
- Cools, N.; De Vos, B. Part X: Sampling and Analysis of Soil. Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests. Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020. Version 2020-1. pp. 1–29. Available online: https://www.icp-forests.org/pdf/manual/2020/ICP_Manual_part10_2020_Soil_version_2020-1.pdf (accessed on 2 October 2023).
- Tedersoo, L.; Mikryukov, V.; Anslan, S.; Bahram, M.; Khalid, A.N.; Corrales, A.; Agan, A.; Vasco-Palacios, A.-M.; Saitta, A.; Antonelli, A.; et al. The Global Soil Mycobiome consortium dataset for boosting fungal diversity research. Fungal Divers. 2021, 111, 573–588. [Google Scholar] [CrossRef]
- ISO 10390; Soil Quality—Determination of pH. ISO (International Standards Organization): Geneva, Switzerland, 2005.
- ISO 10694; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). ISO: Geneva, Switzerland, 1995.
- ISO 11261; Soil Quality—Determination of Total Nitrogen. Modified Kjeldahl Method. ISO: Geneva, Switzerland, 1995.
- Setia, R.; Verma, S.L.; Marschner, P. Measuring microbial biomass carbon by direct extraction—Comparison with chloroform fumigation-extraction. Eur. J. Soil Biol. 2012, 53, 103–106. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Tedersoo, L.; Lindahl, B. Fungal identification biases in microbiome projects. Environ. Microbiol. Rep. 2016, 8, 774–779. [Google Scholar] [CrossRef]
- Anslan, S.; Bahram, M.; Hiiesalu, I.; Tedersoo, L. PipeCraft: Flexible open-source toolkit for bioinformatics analysis of custom high-throughput amplicon sequencing data. Mol. Ecol. Resour. 2017, 17, 234–240. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Nilsson, R.H.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- SAS Institute. The SAS System for Windows, version 9.4; SAS Institute: Cary, NC, USA, 2016. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Tedersoo, L.; Mikryukov, V.; Zizka, A.; Bahram, M.; Hagh-Doust, N.; Anslan, S.; Prylutskyi, O.; Delgado-Baquerizo, M.; Maestre, F.T.; Pärn, J.; et al. Towards understanding diversity, endemicity and global change vulnerability of soil fungi. bioRxiv 2022. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: New York, NY, USA, 2022. [Google Scholar]
- Inkscape Project. Inkscape. 2020. Available online: https://inkscape.org (accessed on 1 December 2023).
- Brewer, T.E.; Aronson, E.L.; Arogyaswamy, K.; Billings, S.A.; Botthoff, J.K.; Campbell, A.N.; Dove, N.C.; Fairbanks, D.; Gallery, R.E.; Hart, S.C.; et al. Ecological and genomic attributes of novel bacterial taxa that thrive in subsurface soil horizons. mBio 2019, 10, e01318-19. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.M.; Kim, H.M.; Jung, J.Y.; Nam, S.; Ju, H.T.; Kim, M.; Lee, Y.K. Distinct taxonomic and functional profiles of the microbiome associated with different soil horizons of a moist tussock tundra in Alaska. Front. Microbiol. 2019, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, G.; Zhang, X.; Wang, Q.; Ge, J.; Liu, S. Changes in nitrogen-cycling microbial communities with depth in temperate and subtropical forest soils. Appl. Soil Ecol. 2018, 124, 218–228. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in microbial community composition and function by soil depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.G.; Ding, Z.J.; Tang, M.; Zhu, B. Changes in soil total, microbial and enzymatic C-N-P contents and stoichiometry with depth and latitude in forest ecosystems. Sci. Total Environ. 2022, 816, 151583. [Google Scholar] [CrossRef]
- Liu, Z.X.; Gu, H.D.; Yao, Q.; Jiao, F.; Liu, J.J.; Jin, J.; Liu, X.B.; Wang, G.H. Microbial communities in the diagnostic horizons of agricultural Isohumosols in northeast China reflect their soil classification. Catena 2022, 216, 106430. [Google Scholar] [CrossRef]
- Ren, G.D.; Wang, G.F.; Ma, Y. Research progresses on relationship between plant root exudates and soil-borne diseases. Soils 2021, 53, 229–235. (In Chinese) [Google Scholar] [CrossRef]
- Fischer, D.; Uksa, M.; Tischler, W.; Kautz, T.; Köpke, U.; Schloter, M. Abundance of ammonia oxidizing microbes and denitrifiers in different soil horizons of an agricultural soil in relation to the cultivated crops. Biol. Fertil. Soils 2013, 49, 1243–1246. [Google Scholar] [CrossRef]
- Sharififar, A.; Minasny, B.; Arrouays, D.; Boulonne, L.; Chevallier, T.; van Deventer, P.; Field, D.J.; Gomez, C.; Jang, H.-J.; Jeon, S.-H.; et al. Chapter Four—Soil inorganic carbon, the other and equally important soil carbon pool: Distribution, controlling factors, and the impact of climate change. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2023; Volume 178, pp. 165–231. [Google Scholar] [CrossRef]
- Hsiao, C.-J.; Sassenrath, G.F.; Zeglin, L.H.; Hettiarachchi, G.M.; Rice, C.W. Vertical changes of soil microbial properties in claypan soils. Soil Biol. Biochem. 2018, 121, 154–164. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Chang. Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Shi, L.; Marshall, M.R.; Kuzyakov, Y.; Blagodatskaya, E.; Zang, H. Strong priming of soil organic matter induced by frequent input of labile carbon. Soil Biol. Biochem. 2021, 152, 108069. [Google Scholar] [CrossRef]
- Osterholz, W.R.; Rinot, O.; Shaviv, A.; Linker, R.; Liebman, M.; Sanford, G.; Strock, J.; Castellano, M.J. Predicting gross nitrogen mineralization and potentially mineralizable nitrogen using soil organic matter properties. Soil Sci. Soc. Am. J. 2017, 81, 1115–1126. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Długosz, J.; Frąc, M.; Gryta, A.; Breza-Boruta, B. Enzymatic activity and functional diversity of soil microorganisms along the soil profile—A matter of soil depth and soil-forming processes. Geoderma 2022, 416, 115779. [Google Scholar] [CrossRef]
- Crowther, T.W.; Van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- He, L.; Rodrigues, J.L.M.; Soudzilovskaia, N.A.; Barceló, M.; Olsson, P.A.; Song, C.; Tedersoo, L.; Yuan, F.; Yuan, F.; Lipson, D.A.; et al. Global biogeography of fungal and bacterial biomass carbon in topsoil. Soil Biol. Biochem. 2020, 151, 108024. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J. Cross-biome drivers of soil bacterial alpha diversity on a worldwide scale. Ecosystems 2019, 22, 1220–1231. [Google Scholar] [CrossRef]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Joergensen, R.G. Stoichiometry of the soil microbial biomass in response to amendments with varying C/N/P/S ratios. Biol. Fertil. Soils 2019, 55, 265–274. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zhang, C.; Wang, H.; Fu, X.; Chen, F.; Wan, S.; Sun, X.; Wen, X.; Wang, J. Understory vegetation plays the key role in sustaining soil microbial biomass and extracellular enzyme activities. Biogeosciences 2018, 15, 4481–4494. [Google Scholar] [CrossRef]
- Hicks, L.C.; Lajtha, K.; Rousk, J. Nutrient limitation may induce microbial mining for resources from persistent soil organic matter. Ecology 2021, 102, e03328. [Google Scholar] [CrossRef]
- Ko, D.; Yoo, G.; Yun, S.T.; Jun, S.C.; Chung, H. Bacterial and fungal community composition across the soil depth profiles in a fallow field. J. Ecol. Environ. 2017, 41, 34. [Google Scholar] [CrossRef]
- Tyukavina, O.N.; Evdokimov, V.N. Pinus sylvestris Root System in the North Taiga Zone (Кoрневая система сoсны oбыкнoвеннoй в услoвиях северoтаежнoй зoны). For. J. 2016, 1, 55–65. (In Russian) [Google Scholar] [CrossRef]
- Finér, L.; Helmisaari, H.-S.; Lõhmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, T.; Godbold, D.; Grebenc, T.; Konôpka, B.; et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Buraczyk, W.; Kapuścińska, M. Effects of pruning of vertical roots on growth of one-year Scots pine (Pinus sylvestris L.) seedlings in the first year after transplanting. Folia For. Pol. Ser. A 2010, 52, 26–32. [Google Scholar]
- Zhiyanski, M. Seasonal dynamics of fine root biomass in selected forest stands. Silva Balc. 2014, 15, 5–15. [Google Scholar]
- Clemmensen, K.E.; Durling, M.K.; Michelsen, A.; Hallin, S.; Finlay, R.D.; Lindahl, B.D. A tipping point in carbon storage when forest expands into tundra is related to mycorrhizal recycling of nitrogen. Ecol. Lett. 2021, 24, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.W.; Heckman, K.; Kolka, R.; Kennedy, P.G. Melanin mitigates the accelerated decay of mycorrhizal necromass with peatland warming. Ecol. Lett. 2019, 22, 498–505. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Tansley insight. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Prada-Salcedo, L.D.; Goldmann, K.; Heintz-Buschart, A.; Reitz, T.; Wambsganss, J.; Bauhus, J.; Buscot, F. Fungal guilds and soil functionality respond to tree community traits rather than to tree diversity in European forests. Mol. Ecol. 2020, 30, 572–591. [Google Scholar] [CrossRef] [PubMed]
- Persoh, D.; Stolle, N.; Brachmann, A.; Begerow, D.; Rambold, G. Fungal guilds are evenly distributed along a vertical spruce forest soil profile while individual fungi show pronounced niche partitioning. Mycol. Prog. 2018, 17, 925–939. [Google Scholar] [CrossRef]







| Horizon | Depth (cm) | Particle Size (%) | ||
|---|---|---|---|---|
| ˂2 µm | 2–63 µm | 63–2000 µm | ||
| OL | −4–−3 | - | - | - |
| OFH | −3–0 | - | - | - |
| E | 0–8 | 1.7 | 3.1 | 95.2 |
| B1 | 8–28 | 0.7 | 2.4 | 96.9 |
| B2 | 28–48 | 0.5 | 0.8 | 98.7 |
| B3g | 48–120 | 0.8 | 0.4 | 98.8 |
| B4g | 120–170 | 1.2 | 0.4 | 98.4 |
| B5gCg | 170–200 | 1.2 | 0.5 | 98.3 |
| Soil Depth (cm) | pHCaCl2 | Concentration (g kg−1) | SOC/STN (C:N) | |||
|---|---|---|---|---|---|---|
| STN | STC | SOC | SIC | |||
| 0–5 | 3.62 ± 0.87 a | 12.5 ± 6.86 | 393.7 ± 68.18 | 358.5 ± 60.68 | 35.2 ± 17.32 | 28.67 |
| 10–15 | 4.30 ± 0.25 ab | 0.41 ± 0.176 d | 10.28 ± 2.58 c | 9.68 ± 2.42 c | 0.70 ± 0.48 ab | 23.42 |
| 20–25 | 4.90 ± 0.10 bcd | 0.29 ± 0.116 bcd | 5.88 ± 1.80 b | 5.22 ± 1.87 b | 0.66 ± 0.36 ab | 18.33 |
| 50–55 | 4.95 ± 0.13 bcd | 0.19 ± 0.292 ab | 3.02 ± 0.29 a | 2.50 ± 0.31 a | 0.46 ± 0.35 ab | 13.21 |
| 100–105 | 5.08 ± 0.12 d | 0.08 ± 0.009 ab | 2.40 ± 0.14 a | 2.10 ± 0.27 a | 0.36 ± 0.28 ab | 25.61 |
| 150–155 | 4.89 ± 0.21 bcd | 0.06 ± 0.035 a | 3.08 ± 0.49 a | 2.38 ± 0.51 a | 0.72 ± 0.19 b | 43.59 |
| 195–200 | 4.79 ± 0.44 bcd | 0.03 ± 0.014 a | 3.22 ± 0.36 a | 2.72 ± 0.23 a | 0.58 ± 0.51 ab | 98.55 |
| F value | 5.008 | 5.216 | 26.34 | 26.76 | 0.722 | - |
| p value | 0.00616 | 0.00222 | 0.00000000518 | 0.00000000443 | 0.614 | - |
| Soil Depth (cm) | Total Number of Sequence Reads Per Sample | Number of Fungal OTUs | Observed Richness of All Fungi | Shannon Index of All Fungi |
|---|---|---|---|---|
| 0–5 | 15,737 | 1118 | 373 ± 30 | 4.042 ± 0.135 |
| 10–15 | 15,115 | 1030 | 343 ± 150 | 4.268 ± 0.230 |
| 20–25 | 13,994 | 1112 | 371 ± 140 | 4.600 ± 0.206 |
| 50–55 | 17,231 | 1299 | 433 ± 91 | 4.791 ± 0.181 |
| 100–105 | 19,774 | 1338 | 446 ± 133 | 4.721 ± 0.058 |
| 150–155 | 15,699 | 1151 | 384 ± 102 | 4.384 ± 0.069 |
| 195–200 | 17,428 | 1109 | 370 ± 86 | 4.155 ± 0.141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankuda, J.; Sivojienė, D.; Armolaitis, K.; Jakutis, A.; Aleinikovienė, J.; Drapanauskaitė, D.; Marozas, V.; Mishcherikova, V.; Stakėnas, V.; Mikryukov, V.; et al. Soil Fungi and Soil Organic Carbon Stocks in the Profile of a Forest Arenosol. Diversity 2024, 16, 66. https://doi.org/10.3390/d16010066
Ankuda J, Sivojienė D, Armolaitis K, Jakutis A, Aleinikovienė J, Drapanauskaitė D, Marozas V, Mishcherikova V, Stakėnas V, Mikryukov V, et al. Soil Fungi and Soil Organic Carbon Stocks in the Profile of a Forest Arenosol. Diversity. 2024; 16(1):66. https://doi.org/10.3390/d16010066
Chicago/Turabian StyleAnkuda, Jelena, Diana Sivojienė, Kęstutis Armolaitis, Audrius Jakutis, Jūratė Aleinikovienė, Donata Drapanauskaitė, Vitas Marozas, Valeriia Mishcherikova, Vidas Stakėnas, Vladimir Mikryukov, and et al. 2024. "Soil Fungi and Soil Organic Carbon Stocks in the Profile of a Forest Arenosol" Diversity 16, no. 1: 66. https://doi.org/10.3390/d16010066
APA StyleAnkuda, J., Sivojienė, D., Armolaitis, K., Jakutis, A., Aleinikovienė, J., Drapanauskaitė, D., Marozas, V., Mishcherikova, V., Stakėnas, V., Mikryukov, V., & Tedersoo, L. (2024). Soil Fungi and Soil Organic Carbon Stocks in the Profile of a Forest Arenosol. Diversity, 16(1), 66. https://doi.org/10.3390/d16010066