Using Impedance Measurements to Characterize Surface Modified with Gold Nanoparticles
Abstract
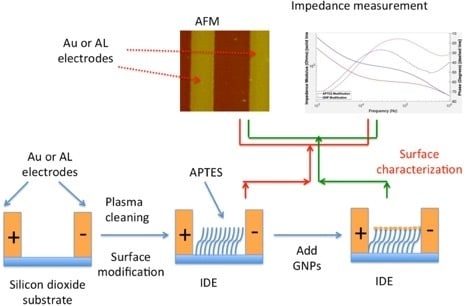
:1. Introduction
2. Methods
2.1. Basic Impedance Tests
2.2. Synthesis and Surface Functionalization of GNPs
2.3. APTES Modification
2.4. MUA-GNPs/APTES Modified IDEs Binding
2.5. Gold Electrode Surface Modification
3. Results
3.1. Gold Nanoparticles Synthesis and Surface Modification
3.2. Effect of Oxygen Plasma on Electrodes and Impedance Measurements
3.3. Impedance Measurements of GNPs on Gold and Aluminum IDEs
3.4. Impedance Measurements of GNPs on Gold IDEs
3.5. Characterizing the Distribution of GNPs on IDEs
3.6. Oversaturation of APTES
3.7. Effect of Clumped GNPs and MUA Surface Modification
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Etzioni, R.; Urban, N.; Ramsey, S.; McIntosh, M.; Schwartz, S.; Reid, B.; Radich, J.; Anderson, G.; Hartwell, L. The case for early detection. Nat. Rev. Cancer 2003, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Cary, K.C.; Cooperberg, M.R. Biomarkers in prostate cancer surveillance and screening: Past, present, and future. Ther. Adv. Urol. 2013, 5, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Lauks, I.R. Microfabricated Biosensors and Microanalytical Systems for Blood Analysis. Acc. Chem. Res. 1998, 31, 317–324. [Google Scholar] [CrossRef]
- Mascini, M.; Tombelli, S. Biosensors for biomarkers in medical diagnostics. Biomarkers 2008, 13, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Mirkin, C.A. Chip-based scanometric detection of mercuric ion using DNA-functionalized gold nanoparticles. Anal. Chem. 2008, 80, 6805–6808. [Google Scholar] [CrossRef] [PubMed]
- JLee, S.; Ulmann, P.A.; Han, M.S.; Mirkin, C.A. A DNA-Gold nanoparticle-based colorimetric competition assay for the detection of cysteine. Nano Lett. 2008, 8, 529–533. [Google Scholar]
- Mehta, B.; Li, Z.; Zaghloul, M. Optical Bio Sensor Using Graphene Nano Ribbons. In Proceedings of the 2011 International Semiconductor Device Research Symposium (ISDRS), College Park, MD, USA, 7–9 December 2011. [Google Scholar]
- Rodriguez, M.C.; Kawde, A.-N.; Wang, J. Aptamer biosensor for label-free impedance spectroscopy detection of proteins based on recognition-induced switching of the surface charge. Chem. Commun. 2005, 14, 4267–4269. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Z.; Yang, G.; Li, J.; Li, M.; Liu, J.; Huang, X. Electrical nanogap devices for biosensing For detecting substances that are invisible to the human eye or nose. Mater. Today 2010, 13, 28–41. [Google Scholar] [CrossRef]
- Su, M.; Li, S.; Dravida, V.P. Microcantilever resonance-based DNA detection with nanoparticle probes. Appl. Phys. Lett. 2003, 82, 3562–3564. [Google Scholar] [CrossRef]
- Moulin, A.M.; O’Shea, S.J.; Welland, E.M. Microcantilever-based biosensors. Ultramicroscopy 2000, 82, 23–31. [Google Scholar] [CrossRef]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Motesharei, K.; Dancil, K.P.; Sailor, M.J.; Ghadiri, M.R. A Porous Silicon-Based Optical Interferometric Biosensor. Science 1997, 278, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, R.; Grattarola, M.; Butt, H.J.; Skládal, P. Micromechanical cantilever-based biosensors. Sens. Actuators B Chem. 2001, 79, 115–126. [Google Scholar] [CrossRef]
- Baptista, P.; Pereira, E.; Eaton, P.; Doria, G.; Miranda, A.; Gomes, I.; Quaresma, P.; Franco, R. Gold nanoparticles for the development of clinical diagnosis methods. Anal. Bioanal. Chem. 2008, 391, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, P.; Mackay, S.; Wishart, D.; Chen, J. Simulations and Design of Microfabricated Interdigitated Electrodes for Use in a Gold Nanoparticle Enhanced Biosensor. In Proceedings of the 2016 IEEE 38th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 299–302. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 6, 1–6. [Google Scholar]
- Huang, C.C.; Huang, Y.F.; Cao, Z.; Tan, W.; Chang, H.T. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal. Chem. 2005, 77, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Mackay, S.; Wishart, D.; Xing, J.Z.; Chen, J. Developing trends in aptamer-based biosensor devices and their applications. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Niazi, J.H.; Kallempudi, S.; Gurbuz, Y. Label-free capacitive biosensor for sensitive detection of multiple biomarkers using gold interdigitated capacitor arrays. Biosens. Bioelectron. 2010, 25, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; Muñiz, D.; Rodríguez, Á. Fabrication of flexible interdigitated μ-electrodes (FIDμEs) for the development of a conductimetric immunosensor for atrazine detection based on antibodies labelled with gold nanoparticles. Microelectron. Eng. 2010, 87, 167–173. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Veselinovic, M.; Yang, L.; Geiss, B.J.; Dandy, D.S.; Chen, T. A sensitive DNA capacitive biosensor using interdigitated electrodes. Biosens. Bioelectron. 2017, 87, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.; Huang, Y.; Wang, X.; Burt, J.P.H. Positive and negative dielectrophoretic collection of colloidal particles using interdigitated castellated microelectrodes. J. Phys. D Appl. Phys. 2000, 25, 881–888. [Google Scholar] [CrossRef]
- Van Gerwen, P.; Laureys, W.; Huyberechts, G.; de Baeck, M.; Baert, K.; Suis, J.; Varlan, A.; Sansen, W.; Hermans, L.; Mertens, R. Nanoscaled interdigitated electrode arrays for biochemical sensors. Sens. Actuators B Chem. 1998, 49, 73–80. [Google Scholar] [CrossRef]
- Cecchet, F.; Marcaccio, M.; Margotti, M.; Paolucci, F.; Rapino, S.; Rudolf, P. Redox mediation at 11-mercaptoundecanoic acid self-assembled monolayers on gold. J. Phys. Chem. B 2006, 110, 2241–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamborini, F.P.; Crooks, R.M. Corrosion Passivation of Gold by n-Alkanethiol Self-Assembled Monolayers: Effect of Chain Length and End Group. Langmuir 1998, 14, 3279–3286. [Google Scholar] [CrossRef]
- Kim, T.; Ye, Q.; Sun, L.; Chan, K.C.; Crooks, R.M. Polymeric self-assembled monolayers. 5. Synthesis and characterization of omega-functionalized, self-assembled diacetylenic and polydiacetylenic monolayers. Langmuir 1996, 12, 6065–6073. [Google Scholar] [CrossRef]
- Jordan, C.E.; Frutos, A.G.; Thiel, A.J.; Corn, R.M. Surface plasmon resonance imaging measurements of DNA hybridization adsorption and streptavidin/DNA multilayer formation at chemically modified gold surfaces. Anal. Chem. 1997, 69, 4939–4947. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Liu, Z.; Wei, G.; Wang, L.; Sun, L. Immobilization of DNA on 11-mercaptoundecanoic acid-modified gold (111) surface for atomic force microscopy imaging. Microsc. Res. Tech. 2005, 68, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E.D. Spontaneously Organized Molecular Assemblies. 4. Structural Characterization of n-Alkyl Thiol Monolayers on Gold by Optical Ellipsometry, Infrared Spetcroscopy, and Electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- MacKay, S.; Hermansen, P.; Wishart, D.; Chen, J. Simulations of interdigitated electrode interactions with gold nanoparticles for impedance-based biosensing applications. Sensors 2015, 15, 22192–22208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, N.; Yu, H.; Niu, Y.; Sun, C. Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 2005, 67, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, G.N.; Farkas, B.; Romano, I.; Diaspro, A.; Beke, S. Nanocomposite scaffold fabrication by incorporating gold nanoparticles into biodegradable polymer matrix: Synthesis, characterization, and photothermal effect. Mater. Sci. Eng. C 2015, 56, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Tang, T.; Harrison, D.J.; Lee, W.E.; Jemere, A.B. A regenerating ultrasensitive electrochemical impedance immunosensor for the detection of adenovirus. Biosens. Bioelectron. 2015, 68, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Kane, V.; Mulvaney, P. Double-Layer Interactions between Self-Assembled Monolayers of ω -Mercaptoundecanoic Acid on Gold Surfaces. Langmuir 1998, 7463, 3303–3311. [Google Scholar] [CrossRef]
- Smalley, J.F.; Chalfant, K.; Feldberg, S.W.; Nahir, T.M.; Bowden, E.F. An indirect laser-induced temperature jump determination of the surface pK(a) of 11-mercaptoundecanoic acid monolayers self-assembled on gold. J. Phys. Chem. B 1999, 103, 1676–1685. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited.pdf. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Murray, R.W.; Feldberg, S.W. Quantized Capacitance Charging of Monolayer-Protected Au Clusters. J. Phys. Chem. B 1998, 102, 9898–9907. [Google Scholar] [CrossRef]
- Golub, A.A.; Zubenko, A.I.; Zhmud, B.V. γ-APTES Modified Silica Gels: The Structure of the Surface Layer. J. Colloid Interface Sci. 1996, 179, 482–487. [Google Scholar] [CrossRef]
- Kim, T.; Lee, C.H.; Joo, S.W.; Lee, K. Kinetics of gold nanoparticle aggregation: Experiments and modeling. J. Colloid Interface Sci. 2008, 318, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective Colorimetric Detection of Polynucleotides Based on the Distance-Dependent Optical Properties of Gold Nanoparticles. Science 1997, 277, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chiuman, W.; Brook, M.A.; Li, Y. Simple and rapid colorimetric biosensors based on DNA aptamer and noncrosslinking gold nanoparticle aggregation. ChemBioChem 2007, 8, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rothberg, L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 14036–14039. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- ACrumbliss, L.; Perine, S.C.; Stonehuerner, J.; Tubergen, K.R.; Zhao, J.; Henkens, R.W. Colloidal gold as a biocompatible immobilization matrix suitable for the fabrication of enzyme electrodes by electrodeposition. Biotechnol. Bioeng. 1992, 40, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Whitesides, G.M. Formation of monolayers by the coadsorption of thiols on gold: Variation in the head group, tail group, and solvent. J. Am. Chem. Soc. 1989, 111, 7155–7164. [Google Scholar] [CrossRef]
- Schmidt, R.; Zhao, T.; Green, J.-B.; Dyer, D.J. Photoinitiated Polymerization of Styrene from Self-Assembled Monolayers on Gold. Langmuir 2002, 18, 1281–1287. [Google Scholar] [CrossRef]






| Baseline Tests | Average Impedance Magnitude (Ohms) | Standard Deviation (Ohms) | Coefficient of Variation |
|---|---|---|---|
| 10 kHz | 869.5 | 143.9 | 0.165 |
| 100 kHz | 601.5 | 59.5 | 0.0989 |
| 1 MHz | 194.2 | 30.8 | 0.159 |
| Baseline Tests | Average Impedance Magnitude (Ohms) | Standard Deviation (Ohms) | Coefficient of Variation |
|---|---|---|---|
| 10 kHz | 735.9 | 103.9 | 0.141 |
| 100 kHz | 562.6 | 53.7 | 0.0954 |
| 1 MHz | 156.2 | 8.38 | 0.0536 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacKay, S.; Abdelrasoul, G.N.; Tamura, M.; Lin, D.; Yan, Z.; Chen, J. Using Impedance Measurements to Characterize Surface Modified with Gold Nanoparticles. Sensors 2017, 17, 2141. https://doi.org/10.3390/s17092141
MacKay S, Abdelrasoul GN, Tamura M, Lin D, Yan Z, Chen J. Using Impedance Measurements to Characterize Surface Modified with Gold Nanoparticles. Sensors. 2017; 17(9):2141. https://doi.org/10.3390/s17092141
Chicago/Turabian StyleMacKay, Scott, Gaser N. Abdelrasoul, Marcus Tamura, Donghai Lin, Zhimin Yan, and Jie Chen. 2017. "Using Impedance Measurements to Characterize Surface Modified with Gold Nanoparticles" Sensors 17, no. 9: 2141. https://doi.org/10.3390/s17092141
APA StyleMacKay, S., Abdelrasoul, G. N., Tamura, M., Lin, D., Yan, Z., & Chen, J. (2017). Using Impedance Measurements to Characterize Surface Modified with Gold Nanoparticles. Sensors, 17(9), 2141. https://doi.org/10.3390/s17092141