Fluorinated Metal Phthalocyanines: Interplay between Fluorination Degree, Films Orientation, and Ammonia Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Study of Thin Films
2.2. Theoretical Calculations
3. Results and Discussion
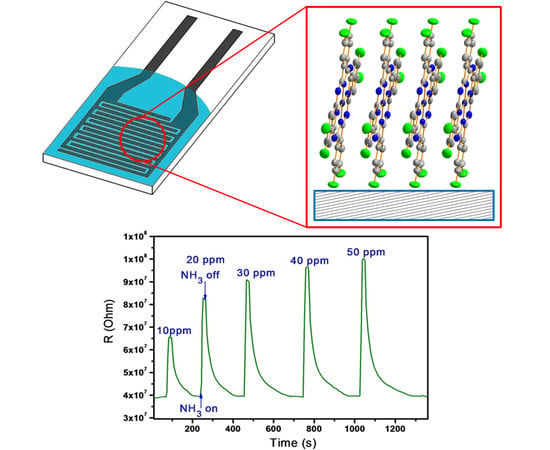
3.1. Experimental Study of the Dependence of Sensing Response on Phthalocyanine Molecular Structure
3.2. Theoretical Study of the Dependence of Sensor Response on the Phthalocyanine Molecular Structure
3.3. Characterization of Thin Films
3.4. Sensor Characteristics of Phthalocyanine Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications: A review. Sens. Actuators B 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.; Eng, S.; Bhattacharya, R.; Rulyak, S.; Hubbard, T.; Putnam, D.; Kearney, D. Breath Ammonia Testing for Diagnosis of Hepatic Encephalopathy. Dig. Dis. Sci. 2005, 50, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Mount, G.H.; Rumberg, B.; Havig, J.; Lamb, B.; Westberg, H.; Yonge, D.; Johson, K.; Kincaid, R. Measurement of atmospheric ammonia at a dairy using differential optical absorption spectroscopy in the mid-ultraviolet. Atmos. Environ. 2002, 36, 1799–1810. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. Development of a polyaniline-based optical ammonia sensor. Sens. Actuators B 2001, 72, 75–79. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Joo, B.-S.; Choi, N.-J.; Lim, J.-O.; Huh, J.-S.; Lee, D.-D. Visible optical sensing of ammonia based on polyaniline film. Sens. Actuators B 2003, 93, 148–152. [Google Scholar] [CrossRef]
- Chen, H.-I.; Hsiao, C.-Y.; Chen, W.-C.; Chang, C.-H.; Chou, T.-C.; Liu, I.-P.; Lin, K.-W.; Liu, W.-C. Characteristics of a Pt/NiO thin film-based ammonia gas sensor. Sens. Actuators B 2018, 256, 962–967. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N., Jr.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Ge, F.; Zhang, G.; Lu, H.; Qiu, L. Solution-Processed Microporous Semiconductor Films for High-Performance Chemical Sensors. Adv. Mater. Interfaces 2016, 3, 1600518. [Google Scholar] [CrossRef]
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef]
- Crowley, K.; Morrin, A.; Hernandez, A.; O’Malley, E.; Whitten, P.G.; Wallace, G.G.; Smytha, M.R.; Killard, A.J. Fabrication of an ammonia gas sensor using inkjet-printed polyaniline nanoparticles. Talanta 2008, 77, 710–717. [Google Scholar] [CrossRef]
- Wannebroucq, A.; Gruntz, G.; Suisse, J.-M.; Nicolas, Y.; Meunier-Prest, R.; Mateos, M.; Toupance, T.; Bouvet, M. New n-type molecular semiconductor-doped insulator (MSDI) heterojunctions combining a triphenodioxazine (TPDO) and the lutetium bisphthalocyanine (LuPc2) for ammonia sensing. Sens. Actuators B 2018, 255, 1694–1700. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhih, A.S.; Krasnov, P.O.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V. Thin films of tetrafluorosubstituted cobalt phthalocyanine: Structure and sensor properties. Appl. Surf. Sci. 2016, 372, 79–86. [Google Scholar] [CrossRef]
- Sukhikh, A.S.; Klyamer, D.D.; Parkhomenko, R.G.; Krasnov, P.O.; Gromilov, S.A.; Hassan, A.K.; Basova, T.V. Effect of fluorosubstitution on the structure of single crystals, thin films and spectral properties of palladium phthalocyanines. Dyes Pigment. 2018, 149, 348–355. [Google Scholar] [CrossRef]
- Hesse, K.; Schlettwein, D. Spectroelectrochemical investigations on the reduction of thin films of hexadecafluorophthalocyaninatozinc (F16PcZn). J. Electroanal. Chem. 1999, 476, 148–158. [Google Scholar] [CrossRef]
- Engel, M.K. Single-Crystal Structures of Phthalocyanine Complexes and Related Macrocycles. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2003; Volume 20, pp. 1–242. ISBN 0-12-39322. [Google Scholar]
- Schollhorn, B.; Germain, J.P.; Pauly, A.; Maleysson, C.; Blanc, J.P. Influence of peripheral electron-withdrawing substituents on the conductivity of zinc phthalocyanine in the presence of gases. Part 1: Reducing gases. Thin Solid Films 1998, 326, 245–250. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Shi, M.; Wu, G.; Wang, M.; Huang, J. High gas-sensitivity and selectivity of fluorinated zinc phthalocyanine film to some non-oxidizing gases at room temperature. Thin Solid Films 2005, 489, 257–261. [Google Scholar] [CrossRef]
- Brinkmann, H.; Kelting, C.; Makarov, S.; Tsaryova, O.; Schnurpfeil, G.; Wöhrle, D.; Schlettwein, D. Fluorinated phthalocyanines as molecular semiconductor thin films. Phys. Status Solidi A 2008, 205, 409–420. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 88, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Schaefer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis set for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schaefer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis set of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system, WIREs. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Mayer, I. Bond order and valence—Relations to Mulliken population analysis. Int. J. Quant. Chem. 1984, 26, 151–154. [Google Scholar] [CrossRef]
- Mayer, I. Bond orders and valences in the SCF theory—A comment. Theor. Chim. Acta 1985, 67, 315–322. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Z.; Wu, H.; Guo, L.; He, C.; Wang, B.; Wu, Y. Enhanced NH3-sensing behavior of 2,9,16,23-tetrakis(2,2,3,3-tetrafluoropropoxy) metal(II) phthalocyanine/multi-walled carbon nanotube hybrids: An investigation of the effects of central metals. Carbon 2014, 80, 268–278. [Google Scholar] [CrossRef]
- Tang, Q.; Li, H.; Liu, Y.; Hu, W. High-performance air-stable n-type transistors with an asymmetrical device configuration based on organic single-crystalline submicrometer/nanometer ribbons. J. Am. Chem. Soc. 2006, 128, 14634–14639. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kerp, H.R.; Westerdui, K.T.; van Veen, A.T.; van Faassen, E.E. Quantification and effects of molecular oxygen and water in zinc phthalocyanine layers. J. Mater. Res. 2001, 16, 503–511. [Google Scholar] [CrossRef]
- De Haan, A.; Debliquy, M.; Decroly, A. Influence of atmospheric pollutants on the conductance of phthalocyanine films. Sens. Actuators B Chem. 1999, 57, 69–74. [Google Scholar] [CrossRef]
- Gould, R.D. Structure and electrical conduction properties of phthalocyanine thin films. Coord. Chem. Rev. 1996, 156, 237–274. [Google Scholar] [CrossRef]
- Schlettwein, D.; Graaf, H.; Meyer, J.-P.; Oekermann, T.; Jaeger, N.I. Molecular interactions in thin films of hexadecafluorophthalocyaninatozinc (F16PcZn) as compared to islands of N,N′-dimethylperylene-3,4,9,10-biscarboximide (MePTCDI). J. Phys. Chem. B 1999, 103, 3078–3086. [Google Scholar] [CrossRef]
- Schlettwein, D.; Hesse, K.; Gruhn, N.E.; Lee, P.A.; Nebesny, K.W.; Armstrong, N.R. Electronic energy levels in individual molecules, thin films, and organic heterojunctions of substituted phthalocyanines. J. Phys. Chem. B 2001, 105, 4791–4800. [Google Scholar] [CrossRef]
- Parkhomenko, R.G.; Sukhikh, A.S.; Klyamer, D.D.; Krasnov, P.O.; Gromilov, S.A.; Kadem, B.; Hassan, A.K.; Basova, T.V. Thin films of unsubstituted and fluorinated palladium phthalocyanines: Structure and sensor response toward ammonia and hydrogen. J. Phys. Chem. C 2017, 121, 1200–1209. [Google Scholar] [CrossRef]
- Erk, P.; Hengelsberg, H.; Haddow, M.F.; van Gelder, R. The innovative momentum of crystal engineering. CrystEngComm 2004, 6, 474–483. [Google Scholar] [CrossRef]
- Ballirano, P.; Caminiti, R.; Ercolani, C.; Maras, A.; Orru, M.A. X-ray powder diffraction structure reinvestigation of the α and β forms of cobalt phthalocyanine and kinetics of the α → β phase transition. J. Am. Chem. Soc. 1998, 120, 12798–12807. [Google Scholar] [CrossRef]
- Vergnat, C.; Landais, V.; Legrand, J.-F.; Brinkmann, M. Orienting semiconducting nanocrystals on nanostructured polycarbonate substrates: Impact of substrate temperature on polymorphism and in-plane orientation. Macromolecules 2011, 44, 3817–3827. [Google Scholar] [CrossRef]
- Pandey, P.A.; Rochford, L.A.; Keeble, D.S.; Rourke, J.P.; Jones, T.S.; Beanland, R.; Wilson, N.R. Resolving the nanoscale morphology and crystallographic structure of molecular thin films: F16CuPc on graphene oxide. Chem. Mater. 2012, 24, 1365–1370. [Google Scholar] [CrossRef]
- Yoon, S.M.; Song, H.J.; Hwang, I.-C.; Kim, K.S.; Choi, H.C. Single crystal structure of copper hexadecafluorophthalocyanine (F16CuPc) ribbon. Chem. Commun. 2010, 46, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-I.; Chi, C.-Y.; Chen, W.-C.; Liu, I.-P.; Chang, C.-H.; Chou, T.-C.; Liu, W.-C. Ammonia Sensing Characteristic of a Pt Nanoparticle/Aluminum-Doped Zinc Oxide Sensor. Sens. Actuators B 2018, 267, 145–154. [Google Scholar] [CrossRef]
- Wang, X.; Meng, S.; Tebyetekerwa, M.; Weng, W.; Pionteck, J.; Sun, B.; Qin, Z.; Zhu, M. Nanostructured polyaniline/poly(styrene-butadiene-styrene) composite fiber for use as highly sensitive and flexible ammonia sensor. Synth. Met. 2017, 233, 86–93. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, A.; Nejad, M.B.; Abadi, M.H.S.; Zou, Z.; Zheng, L.R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Lee, K.; Scardaci, V.; Kim, H.-Y.; Hallam, T.; Nolan, H.; Bolf, B.E.; Maltbie, G.S.; Abbott, J.E.; Duesberg, G.S. Highly sensitive, transparent, and flexible gas sensors based on gold nanoparticle decorated carbon nanotubes. Sens. Actuators B 2013, 188, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, B.; Wang, X.; Zhou, X.; Chen, Z.; He, C.; Yu, Z.; Wu, Y. Enhanced NH3-Sensitivity of Reduced Graphene Oxide Modified by Tetra-α-Iso-Pentyloxymetallophthalocyanine Derivatives. Nanoscale Res. Lett. 2015, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, A.; Kumar, A.; Samanta, S.; Joshi, N.; Balouria, V.; Debnath, A.K.; Prasad, R.; Salmi, Z.; Chehimi, M.M.; et al. Bending stress induced improved chemiresistive gas sensing characteristics of flexible cobalt-phthalocyanine thin films. Appl. Phys. Lett. 2013, 102, 132107. [Google Scholar] [CrossRef]









| Time, s | CoPc | CoPcF4 | CoPcF16 | ZnPc | ZnPcF4 | ZnPcF16 | CuPc | CuPcF4 | CuPcF16 |
|---|---|---|---|---|---|---|---|---|---|
| Response | 15 | 20 | 10 | 10 | 25 | 15 | 10 | 15 | 10 |
| Recovery | 120 | 130 | 160 | 90 | 110 | 85 | 80 | 95 | 80 |
| Aggregate | Eb, eV | Bond Order | d, Å | q(NH3), e |
|---|---|---|---|---|
| CoPc····NH3 | −1.14 | 0.484 | 2.153 | 0.243 |
| CoPcF4····NH3 | −1.16 | 0.486 | 2.152 | 0.245 |
| CoPcF16····NH3 | −1.20 | 0.491 | 2.151 | 0.250 |
| ZnPc····NH3 | −1.06 | 0.402 | 2.159 | 0.214 |
| ZnPcF4····NH3 | −1.08 | 0.405 | 2.156 | 0.216 |
| ZnPcF16····NH3 | −1.14 | 0.414 | 2.151 | 0.223 |
| CuPc····NH3 | −0.62 | 0.291 | 2.330 | 0.156 |
| CuPcF4····NH3 | −0.63 | 0.293 | 2.329 | 0.158 |
| CuPcF16····NH3 | −0.68 | 0.302 | 2.322 | 0.164 |
| Sensing Layer | Concentration Range, ppm | Minimal Investigated Concentration, ppm | Response/ Recovery Time, s | Temperature Range, °C | Ref. |
|---|---|---|---|---|---|
| Metal Oxides | |||||
| Pt/NiO | 1–1000 | 0.01 | 15/76 (350 °C, 1000 ppm) | 200–350 | [7] |
| Pt Nanoparticle/Aluminum-Doped Zinc Oxide | 1–1000 | 1 | 24/4 (350 °C, 1000 ppm) | 200–350 | [48] |
| Conducting Polymers | |||||
| Polyaniline/poly(styrene-butadiene-styrene) | 0.1–100 | 0.1 | ≤13 (100 ppm)/-- | Room temperature | [49] |
| Flexible polyaniline films | 50–150 | 50 | 40 (50 ppm)/-- | Room temperature | [50] |
| Carbon-Containing Nanomaterials and Phthalocyanines | |||||
| AuNPs/SWNT | 0.25–6 | 0.255 | 20 (0.4 ppm)/-- | Room temperature | [51] |
| rGO modified with metal tetra-α-iso-pentyloxyme-tallophthalocyanines (CuPc, NiPc, PbPc) | 0.4–400 | 0.4 | CuPc/rGO 364/115 NiPc/rGO 200/264 PbPc/rGO 248/331 (0.8 ppm) | Room temperature | [52] |
| CoPc on a flexible polyethylene terephthalate substrate | 5–50 | 5 | 25/156 (20 ppm) | Room temperature | [53] |
| ZnPcF4 | 0.1–50 | 0.1 | 25/110 (10 ppm) | Room temperature | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated Metal Phthalocyanines: Interplay between Fluorination Degree, Films Orientation, and Ammonia Sensing Properties. Sensors 2018, 18, 2141. https://doi.org/10.3390/s18072141
Klyamer D, Sukhikh A, Gromilov S, Krasnov P, Basova T. Fluorinated Metal Phthalocyanines: Interplay between Fluorination Degree, Films Orientation, and Ammonia Sensing Properties. Sensors. 2018; 18(7):2141. https://doi.org/10.3390/s18072141
Chicago/Turabian StyleKlyamer, Darya, Aleksandr Sukhikh, Sergey Gromilov, Pavel Krasnov, and Tamara Basova. 2018. "Fluorinated Metal Phthalocyanines: Interplay between Fluorination Degree, Films Orientation, and Ammonia Sensing Properties" Sensors 18, no. 7: 2141. https://doi.org/10.3390/s18072141
APA StyleKlyamer, D., Sukhikh, A., Gromilov, S., Krasnov, P., & Basova, T. (2018). Fluorinated Metal Phthalocyanines: Interplay between Fluorination Degree, Films Orientation, and Ammonia Sensing Properties. Sensors, 18(7), 2141. https://doi.org/10.3390/s18072141