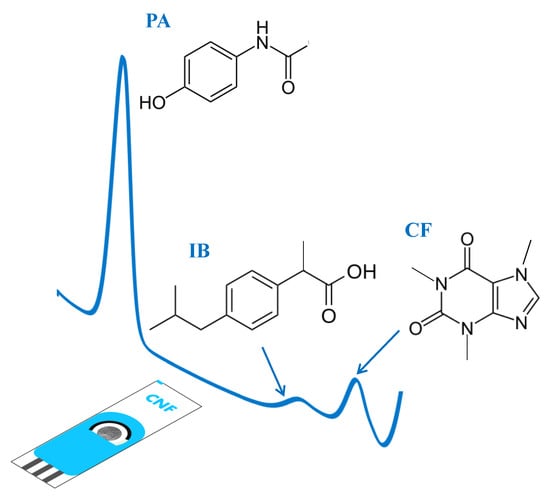
Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standard Solutions
2.2. Apparatus
2.3. Differential Pulse Voltammetry (DPV) Measurements
2.4. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Measurements
3. Results and Discussion
3.1. Optimization of Condition Media and the Potential Range
3.2. Repeatability and Reproducibility
3.3. Sensitivity, Linearity, Limit of Detection (LOD), and Limit of Quantification (LOQ)
3.4. Method Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bottoni, P.; Caroli, S.; Barra Caracciolo, A. Pharmaceuticals as priority water contaminants. Toxicol. Environ. Chem. 2010, 92, 549–565. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239–240, 40–47. [Google Scholar]
- World Health Organization. Pharmaceuticals in Drinking-Water; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Brunton, L.L.; Lazo, J.S.; Parker, K.L. Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; Mc Graw Hill: New York, NY, USA, 2006. [Google Scholar]
- Ternes, T.A. Analytical methods for the determination of pharmaceuticals in aqueous environmental samples. TrAC-Trends Anal. Chem. 2001, 20, 419–434. [Google Scholar] [CrossRef]
- Petrovic, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid chromatography-tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ryu, H.D.; Chung, E.G.; Kim, Y.; Lee, J.K. A review of analytical procedures for the simultaneous determination of medically important veterinary antibiotics in environmental water: Sample preparation, liquid chromatography, and mass spectrometry. J. Environ. Manag. 2018, 217, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, R.; Radhapyari, K.; Jadon, N.; Agarwal, S. Voltammetric techniques for the assay of pharmaceuticals—A review. Anal. Biochem. 2011, 408, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Kachoosangi, R.T.; Wildgoose, G.C.; Compton, R.G. Sensitive adsorptive stripping voltammetric determination of paracetamol at multiwalled carbon nanotube modified basal plane pyrolytic graphite electrode. Anal. Chim. Acta 2008, 618, 54–60. [Google Scholar] [CrossRef]
- Ghadimi, H.; Tehrani, R.M.A.; Ali, A.S.M.; Mohamed, N.; Ab Ghani, S. Sensitive voltammetric determination of paracetamol by poly (4-vinylpyridine)/multiwalled carbon nanotubes modified glassy carbon electrode. Anal. Chim. Acta 2013, 765, 70–76. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Chatterjee, S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sens. Actuators B Chem. 2010, 149, 252–258. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Oyama, M.; Bachheti, N. Differential pulse voltammetric determination of paracetamol at nanogold modified indium tin oxide electrode. Electrochem. Commun. 2005, 7, 803–807. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.H.; Lu, H.T.; Zhang, Q. Electrochemical behavior and voltammetric determination of paracetamol on Nafion/TiO2–graphene modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2011, 85, 289–292. [Google Scholar] [CrossRef]
- Goyal, R.N.; Singh, S.P. Voltammetric determination of paracetamol at C60-modified glassy carbon electrode. Electrochim. Acta 2006, 51, 3008–3012. [Google Scholar] [CrossRef]
- ShangGuan, X.; Zhang, H.; Zheng, J. Electrochemical behavior and differential pulse voltammetric determination of paracetamol at a carbon ionic liquid electrode. Anal. Bioanal. Chem. 2008, 391, 1049–1055. [Google Scholar] [CrossRef]
- El Bouabi, Y.; Farahi, A.; Labjar, N.; El Hajjaji, S.; Bakasse, M.; El Mhammedi, M.A. Square wave voltammetric determination of paracetamol at chitosan modified carbon paste electrode: Application in natural water samples, commercial tablets and human urines. Mater. Sci. Eng. B 2016, 58, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hernández, S.I.; Álvarez-Romero, G.A.; Corona-Alvendaño, S.; Páez-Hernández, M.E.; Galán-Vidal, C.A.; Romero-Romo, M. Voltammetric determination of ibuprofen using a carbon paste—Multiwalled carbon nanotube composite electrode. Instrum. Sci. Technol. 2016, 44, 483–494. [Google Scholar] [CrossRef]
- Mekassa, B.; Tessema, M.; Chandravanshi, B.S.; Tefera, M. Square wave voltammetric determination of ibuprofen at poly(l-aspartic acid) modified glassy carbon electrode. IEEE Sens. J. 2018, 18, 37–44. [Google Scholar] [CrossRef]
- Lima, A.B.; Faria, E.O.; Montes, R.H.O.; Cunha, R.R.; Richter, E.M.; Muñoz, R.A.A.; dos Santos, W.T.P. Electrochemical oxidation of ibuprofen and its voltammetric determination at a boron-doped diamond electrode. Electroanalysis 2013, 25, 1585–1588. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Bejinaru, A.A.; Boev, M.; Apetrei, C.; Buzia, O.D. Determination of ibuprofen based on screen-printed electrodes modified with carbon nanofibers. Farmacia 2017, 65, 790–795. [Google Scholar]
- Ören, T.; Anik, Ü. Voltammetric determination of caffeine by using gold nanoparticle-glassy carbon paste composite electrode. Measurement 2017, 106, 26–30. [Google Scholar] [CrossRef]
- Tadesse, Y.; Tadese, A.; Saini, R.C.; Pal, R. Cyclic voltammetric investigation of caffeine at anthraquinone modified carbon paste electrode. Int. J. Electrochem. 2013, 2013, 849327. [Google Scholar] [CrossRef]
- Svorc, L. Determination of caffeine: A comprehensive review on electrochemical methods. Int. J. Electrochem. Sci. 2013, 8, 5755–5773. [Google Scholar]
- Motoc, S.; Manea, F.; Lacob, A.; Martínez-Joaristi, A.; Gascon, J.; Pop, A.; Schoonman, J. Electrochemical selective and simultaneous detection of diclofenac and ibuprofen in aqueous solution using HKUST-1 metal-organic framework-carbon nanofiber composite electrode. Sensors 2016, 16, 1719. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.B.; Torres, L.M.F.C.; Guimaraes, C.F.R.C.; Verly, R.M.; da Silva, L.M.; Carvalho Júnior, A.D.; dos Santos, W.T.P. Simultaneous determination of paracetamol and ibuprofen in pharmaceutical samples by differential pulse voltammetry using a boron-doped diamond electrode. J. Braz. Chem. Soc. 2014, 25, 478–483. [Google Scholar] [CrossRef]
- Lourençao, B.C.; Medeiros, R.A.; Rocha-Filho, R.C.; Mazo, L.H.; Fatibello-Filho, O. Simultaneous voltammetric determination of paracetamol and caffeine in pharmaceutical formulations using a boron-doped diamond electrode. Talanta 2009, 78, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wang, Y.; Kokot, S. Differential pulse stripping voltammetric determination of paracetamol and phenobarbital in pharmaceuticals assisted by chemometrics. Anal. Lett. 2004, 37, 3219–3235. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. New approaches to antimony film screen-printed electrodes using carbon-based nanomaterials substrates. Anal. Chim. Acta 2016, 916, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Determination of 19 sulfonamides in environmental waters by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry (SPE-LC-MS/MS). Talanta 2010, 81, 355–366. [Google Scholar] [CrossRef]
- Serrano, N.; Cetó, X.; Núñez, O.; Aragó, M.; Gámez, A.; Ariño, C.; Díaz-Cruz, J.M. Characterization and classification of Spanish paprika (Capsicum annuum L.) by liquid chromatography coupled to electrochemical detection with screen-printed carbon-based nanomaterials electrodes. Talanta 2018, 189, 296–301. [Google Scholar] [CrossRef]






| Electrode | Paracetamol | Ibuprofen | Caffeine | |||
|---|---|---|---|---|---|---|
| Repeatability (RSD%) | Reproducibility (RSD%) | Repeatability (RSD%) | Reproducibility (RSD%) | Repeatability (RSD%) | Reproducibility (RSD%) | |
| SPCE | 2.2 | 4.4 | 5.2 | 5.5 | 4.2 | 6.5 |
| SPCNTE | 2.4 | 11.0 | 2.9 | 4.2 | 4.4 | 5.8 |
| SPCNFE | 3.2 | 5.1 | 4.1 | 4.6 | 4.7 | 10.2 |
| SPGPHE | 5.2 | 12.8 | 11.0 | 24.0 | 9.1 | 10.9 |
| Electrode | Paracetamol | Ibuprofen | Caffeine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (μA V mg−1 L) | R2 | Linear Range a (mg L−1) | LOD (mg L−1) | Sensitivity (μA V mg−1 L) | R2 | Linear Range a (mg L−1) | LOD (mg L−1) | Sensitivity (μA V mg−1 L) | R2 | Linear Range a (mg L−1) | LOD (mg L−1) | |
| SPCE | 0.056 (0.001) | 0.999 | 2.0–57.8 | 0.6 | 0.0380 (0.0003) | 0.999 | 3.8–100.9 | 1.1 | 0.0366 (0.0004) | 0.997 | 4.8–93.3 | 1.4 |
| SPCNTE | 0.293 (0.005) | 0.996 | 0.4–5.1 | 0.1 | 0.0404 (0.0005) | 0.998 | 1.9–32.0 | 0.6 | 0.0345 (0.0003) | 0.999 | 4.0–93.3 | 1.2 |
| SPCNFE | 2.66 (0.04) | 0.998 | 0.3–5.1 | 0.1 | 0.082 (0.003) | 0.996 | 4.0–23.6 | 1.2 | 0.31 (0.01) | 0.998 | 1.2–6.4 | 0.4 |
| SPGPHE | 2.2 (0.1) | 0.996 | 0.5–3.5 | 0.1 | 0.051 (0.003) | 0.999 | 6.3–17.8 | 1.9 | 0.20 (0.01) | 0.997 | 3.0–8.7 | 0.9 |
| Sensitivity (μA V mg−1 L) | R2 | Linear Range a (mg L−1) | LOD (mg L−1) | |
|---|---|---|---|---|
| Paracetamol | 2.50 (0.05) | 0.996 | 0.09–0.8 | 0.03 |
| Ibuprofen | 0.074 (0.002) | 0.997 | 2.2–10.2 | 0.6 |
| Caffeine | 0.24 (0.01) | 0.999 | 0.2–1.1 | 0.05 |
| Electrode | Technique | Analyte | LOD (mg L−1) | Application | Ref. |
|---|---|---|---|---|---|
| Multiwalled carbon nanotube modified basal plane pyrolytic graphite electrode | AdSV | PA | 0.002 | Drugs | [9] |
| Poly (4-vinylpyridine)/multiwalled carbon nanotubes modified glassy carbon electrode | DPV | PA | 0.0003 | Drugs, urine | [10] |
| Carbon nanotube modified pyrolytic graphite electrode | CV SWV | PA | 0.0004 | Drugs, urine | [11] |
| Nanogold modified indium tin oxide electrode | DPV | PA | 0.03 | Drugs | [12] |
| Nafion/TiO2–graphene modified glassy carbon electrode | CV DPV | PA | 0.03 | Drugs | [13] |
| C60-modified glassy carbon electrode | DPV | PA | 7.6 | Drugs, urine | [14] |
| Carbon ionic liquid electrode | CV DPV | PA | 0.05 | Drugs, urine | [15] |
| Chitosan modified carbon paste electrode | CV SWV | PA | 0.08 | Water samples, drugs, urine | [16] |
| Carbon paste-multiwalled carbon nanotube composite electrode | DPV | IB | 0.6 | Drugs | [17] |
| Poly(L-aspartic acid) modified glassy carbon electrode | CV SWV | IB | 0.03 | Drugs, urine | [18] |
| Boron-doped diamond electrode | CV DPV | IB | 0.8 | Drugs | [19] |
| Screen-printed carbon electrode modified with carbon nanofibers | CV DPV | IB | 0.05 | Drugs | [20] |
| Gold nanoparticle-glassy carbon paste composite electrode | DPV | CF | 0.2 | Beverages | [21] |
| Anthraquinone modified carbon paste electrode | CV SWV | CF | 0.02 | Drugs | [22] |
| All the electrodes cited therein | Various | CF | 9 × 10−5–47.4 | Drugs, urine, serum, beverages | [23](*) |
| HKUST-1 metal-organic framework-carbon nanofiber composite electrode | CV | IB, diclofenac | 0.02 (IB) | Water samples | [24] |
| Boron-doped diamond electrode | DPV | PA, IB | 1.1 (PA) 0.8 (IB) | Drugs | [25] |
| Boron-doped diamond electrode | SWV DPV | PA, CF | 0.07 (PA) 0.007 (CF) | Drugs | [26] |
| Glassy carbon electrode | DPSV | PA, phenobarbital | 0.04 (PA) | Drugs | [27] |
| Screen-printed carbon electrode modified with carbon nanofibers. | DPV | PA, IB, CF | 0.03 (PA) 0.6 (IB) 0.05 (CF) | Water samples | This work |
| Paracetamol | Ibuprofen | Caffeine | |
|---|---|---|---|
| Cdetermined (mg L−1) | 1.7 (0.2) | 19.8 (0.2) | 1.9 (0.2) |
| RSD (%) | 8.93 | 0.96 | 8.63 |
| Relative error (%) | 3.1 | 0.5 | 2.4 |
| Recovery (%) | 103.1 | 99.5 | 97.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, N.; Castilla, Ò.; Ariño, C.; Diaz-Cruz, M.S.; Díaz-Cruz, J.M. Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples. Sensors 2019, 19, 4039. https://doi.org/10.3390/s19184039
Serrano N, Castilla Ò, Ariño C, Diaz-Cruz MS, Díaz-Cruz JM. Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples. Sensors. 2019; 19(18):4039. https://doi.org/10.3390/s19184039
Chicago/Turabian StyleSerrano, Núria, Òscar Castilla, Cristina Ariño, M. Silvia Diaz-Cruz, and José Manuel Díaz-Cruz. 2019. "Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples" Sensors 19, no. 18: 4039. https://doi.org/10.3390/s19184039
APA StyleSerrano, N., Castilla, Ò., Ariño, C., Diaz-Cruz, M. S., & Díaz-Cruz, J. M. (2019). Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples. Sensors, 19(18), 4039. https://doi.org/10.3390/s19184039