Novel Cost-Effective Microfluidic Chip Based on Hybrid Fabrication and Its Comprehensive Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Tools for Microfluidic Chip Fabrication
2.1.1. Materials
2.1.2. Equipment and Small Tools
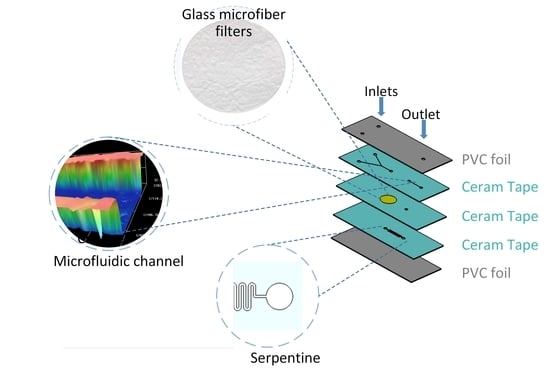
2.2. Methods for Microfluidic Chip Fabrication
2.3. Characterization Techniques
3. Results
3.1. Optical Transmission
3.2. Dielectric Properties
3.3. SEM and Profiler Analysis
3.4. Mechanical Characterization
3.5. Temperature Exposure
3.6. Microfluidic Testing
4. Application Examples
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shuichi Shoji, K.K. Microfluidics: Technologies and Applications; Springer: New York, NY, USA, 2011; Volume XII, p. 344. [Google Scholar]
- Roh, C.; Lee, J.; Kang, C. The Deformation of Polydimethylsiloxane (PDMS) Microfluidic Channels Filled with Embedded Circular Obstacles under Certain Circumstances. Molecules 2016, 21, 798. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Kong, F.; Yeo, J.C.; Yu, L.; Sonam, S.; Dao, M.; Gong, X.; Lim, C.T. Soft tubular microfluidics for 2D and 3D applications. Proc. Natl. Acad. Sci. USA 2017, 114, 10590–10595. [Google Scholar] [CrossRef] [PubMed]
- Ville, M.D.; Coquet, P.; Brunet, P.; Boukherroub, R. Simple and low-cost fabrication of PDMS microfluidic round channels by surface-wetting parameters optimization. Microfluid. Nanofluidics 2011, 12, 953–961. [Google Scholar] [CrossRef]
- Villegas, M.; Cetinic, Z.; Shakeri, A.; Didar, T.F. Fabricating smooth PDMS microfluidic channels from low-resolution 3D printed molds using an omniphobic lubricant-infused coating. Anal. Chim. Acta 2018, 1000, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Shameli, S.M.; Elbuken, C.; Ou, J.; Ren, C.L.; Pawliszyn, J. Fully integrated PDMS/SU-8/quartz microfluidic chip with a novel macroporous poly dimethylsiloxane (PDMS) membrane for isoelectric focusing of proteins using whole-channel imaging detection. Electrophoresis 2011, 32, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.; Galas, J.-C.; Veres, T. Thermoplastic elastomers for microfluidics: Towards a high-throughput fabrication method of multilayered microfluidic devices. Lab Chip 2011, 11, 3193–3196. [Google Scholar] [CrossRef]
- Schianti, J.N.; Cerize, N.P.N.; Oliveira, A.M.; Derenzo, S.; Góngora-Rubio, M.R. 3-D LTCC microfluidic device as a tool for studying nanoprecipitation. J. Phys. Conf. Ser. 2013, 421, 012012. [Google Scholar] [CrossRef] [Green Version]
- Słobodzian, P.; Macioszczyk, J.; Malecha, K.; Golonka, L. A LTCC microwave-microfluidic reactor. In Proceedings of the 2016 21st International Conference on Microwave, Radar and Wireless Communications (MIKON), Krakow, Poland, 9–11 May 2016; pp. 1–4. [Google Scholar]
- Fercher, G.; Smetana, W.; Vellekoop, M.J. Contactless Conductivity Detection in Ceramic Technology for On-Chip Electrophoresis. In Proceedings of the 12 International Conference on Miniaturized Systems for Chemistry and Life Sciences, Sand Diego, CA, USA, 12–16 October 2009; pp. 145–148. [Google Scholar]
- Malecha, K.; Golonka, L.J.; Bałdyga, J.; Jasińska, M.; Sobieszuk, P. Serpentine microfluidic mixer made in LTCC. Sens. Actuators B Chem. 2009, 143, 400–413. [Google Scholar] [CrossRef]
- Jankovic, N.; Radonic, V. A Microwave Microfluidic Sensor Based on a Dual-Mode Resonator for Dual-Sensing Applications. Sensors 2017, 17, 2713. [Google Scholar] [CrossRef]
- Ciobanu, R.-C.; Schreiner, C.; Drug, V.; Schreiner, T.; Antal, D. Sensors in LTCC-technology with embedded microfluidic features, for medical applications. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Turin, Italy, 7–9 May 2015; pp. 407–410. [Google Scholar]
- Malecha, K.; Gancarz, I.; Golonka, L.J. A PDMS/LTCC bonding technique for microfluidic application. J. Micromech. Microeng. 2009, 19, 1–8. [Google Scholar] [CrossRef]
- Malecha, K. The utilization of LTCC-PDMS bonding technology for microfluidic system applications—A simple fluorescent sensor. Microelectron. Int. 2016, 33, 141–148. [Google Scholar] [CrossRef]
- Malecha, K.; Gancarz, I.; Tylus, W. Argon plasma-assisted PDMS–LTCC bonding technique for microsystem applications. J. Micromech. Microeng. 2010, 20, 1–8. [Google Scholar] [CrossRef]
- Luo, J.; Dziubla, T.; Eitel, R. A low temperature co-fired ceramic based microfluidic Clark-type oxygen sensor for real-time oxygen sensing. Sens. Actuators B Chem. 2017, 240, 392–397. [Google Scholar] [CrossRef]
- Vasudev, A.; Kaushik, A.; Jones, K.; Bhansali, S. Prospects of low temperature co-fired ceramic (LTCC) based microfluidic systems for point-of-care biosensing and environmental sensing. Microfluid. Nanofluidics 2013, 14, 683–702. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Wu, S.-Y.; Yang, C.; Restaino, M.; Lin, L. 3D printed microfluidics and microelectronics. Microelectron. Eng. 2018, 189, 52–68. [Google Scholar] [CrossRef]
- Alizadehgiashi, M.; Gevorkian, A.; Tebbe, M.; Seo, M.; Prince, E.; Kumacheva, E. 3D-Printed Microfluidic Devices for Materials Science. Adv. Mater. Technol. 2018, 3, 1800068. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef]
- Rusling, J.F. Developing Microfluidic Sensing Devices Using 3D Printing. ACS Sens. 2018, 3, 522–526. [Google Scholar] [CrossRef]
- Radonic, V.; Birgermajer, S.; Kitic, G. Microfluidic EBG Sensor Based on Phase-Shift Method Realized Using 3D Printing Technology. Sensors 2017, 17, 892. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, J.M.; Liu, Y.; Li, X.; Lv, P.; Jin, D.; Duan, H. A Modular Microfluidic Device via Multimaterial 3D Printing for Emulsion Generation. Sci. Rep. 2018, 8, 4791. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.; Seo, D.; Roy, M.; Han, E.; Candler, R.N.; Seo, S. Capillary Flow in PDMS Cylindrical Microfluidic Channel Using 3-D Printed Mold. J. Microelectromec. Syst. 2016, 25, 238–240. [Google Scholar] [CrossRef]
- Hur, D.; Say, M.G.; Diltemiz, S.E.; Duman, F.; Ersöz, A.; Say, R. 3D Micropatterned All-Flexible Microfluidic Platform for Microwave-Assisted Flow Organic Synthesis. ChemPlusChem 2018, 83, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.; Paydar, O.H.; Candler, R.N. 3D printed molds for non-planar PDMS microfluidic channels. Sens. Actuators A Phys. 2015, 226, 137–142. [Google Scholar] [CrossRef]
- Lim, C.; Koh, K.; Ren, Y.; Chin, J.; Shi, Y.; Yan, Y. Analysis of Liquid–Liquid Droplets Fission and Encapsulation in Single/Two Layer Microfluidic Devices Fabricated by Xurographic Method. Micromachines 2017, 8, 49. [Google Scholar] [CrossRef]
- Zhao, X. Update on Medical Plasticised PVC, 1st ed.; Smithers Rapra Press: Shrewsbury, UK, 2009. [Google Scholar]
- Sastri, V.R. Plastics in Medical Devices: Properties, Requirements and Applications, 1st ed.; Elsevier: Burlington, VT, USA, 2010. [Google Scholar]
- Staff, P.D.L. Chemical Resistance, Volume 1: Thermoplastics, 2nd ed.; William Andrew: New York, NY, USA, 1994. [Google Scholar]
- Yianni, J.P. Making PVC more biocompatible. Med Device Technol. 1995, 6, 20–26. [Google Scholar]
- Franz, M.; Atassi, I.; Maric, A.; Balluch, B.; Weilguni, M.; Smetana, W.; Kluge, C.P.; Radosavljevic, G. Material Characteristics of the LTCC Base Material CeramTape GC. In Proceedings of the 35th International Spring Seminar on Electronics Technology, Bad Aussee, Austria, 9–13 May 2012; pp. 276–281. [Google Scholar]
- Malecha, K.; Remiszewska, E.; Pijanowska, D.G. Technology and application of the LTCC-based microfluidic module for urea determination. Microelectron. Int. 2015, 32, 126–132. [Google Scholar] [CrossRef]
- Smetana, W.; Balluch, B.; Atassi, I.; Gvichiyarahim, K.E.; Gaubitzer, E.; Edetsberger, M.; Koehler, G. A Biological Monitoring Module based on a Ceramic Microfluidic Platform. In Proceedings of the International Conference on Biomedical Electronics and Devices, Porto, Portugal, 14–17 January 2009. [Google Scholar]
- Radonic, V.; Cselyuszka, N.; Crnojevic-Bengin, V.; Kitic, G. Phase-Shift Transmission Line Method for Permittivity Measurement and Its Potential in Sensor Applications. In Electromagnetic Materials, 1st ed.; Han, P.M.-G., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Baker-Jarvis, J.R.; Janezic, M.D.; Riddle, B.F.; Johnk, R.T.; Holloway, C.L.; Geyer, R.G.; Grosvenor, C.A. Measuring the Permittivity and Permeability of Lossy Materials: Solids, Liquids, Metals, Building Materials, and Negative-Index Materials; U.S. Department of Commerce, Technology Administration, National Institute of Standards and Technology: Washington, DC, USA, 2005.
- Herold, K.E.; Rasooly, A. Lab on a Chip Technology: Fabrication and Microfluidics, 1st ed.; Caister Academic Press: Norfolk, UK, 2009. [Google Scholar]
- Finkelstein, S.A. Identifying pollen grains of Typha latifolia, Typha angustifolia, and Typha × glauca. Can. J. Bot. 2003, 81, 985–990. [Google Scholar] [CrossRef]

















| Step 1: Laser cutting | ||
| Parameter | Value | Unit |
| Current | 28 | mA |
| Frequency | 10 | kHz |
| Speed | 15 | mm/s |
| Step 2: Uniaxial press | ||
| Parameter | Value | Unit |
| Pressure | 2200 | kg |
| Temperature | 80 | °C |
| Time | 3 | min |
| Step 3: Plotter cutting | ||
| Parameter | Value | Note |
| Cutting speed—inlet/outlet | 30 cm/s | from range 1–60 |
| Cutting speed—border | 60 cm/s | from range 1–60 |
| Cutting speed—channel | 10 cm/s | from range 1–60 |
| Cutting force—80 µm PVC | 19 | from range 1–38 |
| Cutting force—125 µm PVC | 26 | from range 1–38 |
| Step 4: Lamination | ||
| Parameter | Value | Note |
| Temperature | 120–180 °C | 150 °C if not indicated otherwise |
| Speed | 1 cm/min | from range 1–9 |
| Range | From | To | Step |
|---|---|---|---|
| I | 0.1 µL/min | 1.0 µL/min | 0.1 µL/min |
| II | 1 µL/min | 10 µL/min | 1 µL/min |
| III | 10 µL/min | 100 µL/min | 10 µL/min |
| IV | 100 µL/min | 1 mL/min | 100 µL/min |
| V | 1 mL/min | 15 mL/min | 1 mL/min |
| Technology | Proposed | PDMS | LTCC | 3D Printing | Xurography |
|---|---|---|---|---|---|
| Optical transparency | good | excellent | none can be achieved by bonding of LTCC with other transparent materials | poor | good |
| Mechanical flexibility | flexible | flexible and stretchable | none | none | flexible |
| Channel edge roughness | excellent | good | good | poor | poor |
| Bio-compatibility | good | excellent | excellent | good | good |
| Temperature exposure | up to 120 °C | up to 200 °C properties of PDMS changes with temperature | very high >1000 °C | up to 160 °C | up to 120 °C |
| Possibility to crate complex geometries | excellent | good but requires additional mould and supporting materials | excellent | excellent but requires supporting layer | medium |
| Fabrication time simple/complex geometry | 1–10 min | Couple of hours | >6 h | 10–180 min | 1–10 min |
| Fabrication complexity | simple/medium | complex | complex | simple | simple |
| Technology | Advantages | Disadvantages |
|---|---|---|
| Proposed |
|
|
| PDMS |
|
|
| LTCC |
|
|
| 3D printing |
|
|
| Xurography |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojic, S.P.; Stojanovic, G.M.; Radonic, V. Novel Cost-Effective Microfluidic Chip Based on Hybrid Fabrication and Its Comprehensive Characterization. Sensors 2019, 19, 1719. https://doi.org/10.3390/s19071719
Kojic SP, Stojanovic GM, Radonic V. Novel Cost-Effective Microfluidic Chip Based on Hybrid Fabrication and Its Comprehensive Characterization. Sensors. 2019; 19(7):1719. https://doi.org/10.3390/s19071719
Chicago/Turabian StyleKojic, Sanja P., Goran M. Stojanovic, and Vasa Radonic. 2019. "Novel Cost-Effective Microfluidic Chip Based on Hybrid Fabrication and Its Comprehensive Characterization" Sensors 19, no. 7: 1719. https://doi.org/10.3390/s19071719
APA StyleKojic, S. P., Stojanovic, G. M., & Radonic, V. (2019). Novel Cost-Effective Microfluidic Chip Based on Hybrid Fabrication and Its Comprehensive Characterization. Sensors, 19(7), 1719. https://doi.org/10.3390/s19071719