Layered Double Hydroxide-Modified Organic Electrochemical Transistor for Glucose and Lactate Biosensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. OECT Sensor Preparation
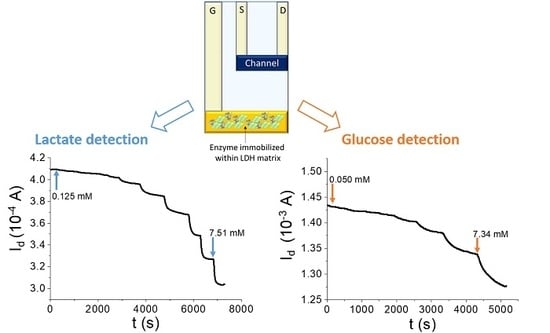
3. Results
3.1. Transistor Configuration and Characterisation
3.2. Modification of the Gate Electrode
3.3. OECT Glucose Sensor
3.4. OECT Lactate Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Gough, D.A.; Andrade, J.D. Enzyme electrodes. Science 1973, 180, 380–384. [Google Scholar] [CrossRef]
- Scheller, F.; Schubert, F.; Pfeiffer, D.; Hintsche, R.; Dransfeld, I.; Renneberg, R.; Wollenberger, U.; Riedel, K.; Pavlova, M.; Kühn, M.; et al. Research and development of biosensors—A review. Analyst 1989, 114, 653–662. [Google Scholar] [CrossRef]
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Reach, G.; Wilson, G.S. Can continuous glucose monitoring be used for the treatment of diabetes. Anal. Chem. 1992, 64, 381A–386A. [Google Scholar] [PubMed]
- Turner, A.P.; Chen, B.; Piletsky, S. In vitro diagnostics in diabetes: Meeting the challenge. Clin. Chem. 1999, 45, 1596–1601. [Google Scholar] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [Green Version]
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.R.; Van Den Berg, A. Lactate biosensors: Current status and outlook. Anal. Bioanal. Chem. 2014, 406, 123–137. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, N.; Strehlitz, B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim. Acta 2008, 160, 15–55. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [Green Version]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Tékus, É.; Kaj, M.; Szabó, E.; Szénási, N.L.; Kerepesi, I.; Figler, M.; Gábriel, R.; Wilhelm, M. Comparison of blood and saliva lactate. Acta Biol. Hung. 2012, 63, 89–98. [Google Scholar]
- Tiongco, R.-E.; Bituin, A.; Arceo, E.; Rivera, N.; Singian, E. Salivary glucose as a non-invasive biomarker of type 2 diabetes mellitus. J. Clin. Exp. Dent. 2018, 10, e902–e907. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable chemical sensors: Present challenges and future prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Yang, S.Y.; Cicoira, F.; Byrne, R.; Benito-Lopez, F.; Diamond, D.; Owens, R.M.; Malliaras, G.G. Electrochemical transistors with ionic liquids for enzymatic sensing. Chem. Commun. 2010, 46, 7972–7974. [Google Scholar] [CrossRef] [Green Version]
- Gualandi, I.; Scavetta, E.; Mariani, F.; Tonelli, D.; Tessarolo, M.; Fraboni, B. All poly(3,4-ethylenedioxythiophene) organic electrochemical transistor to amplify amperometric signals. Electrochim. Acta 2018, 268, 476–483. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F. Organic thin-film transistors for chemical and biological sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Cramer, T.; Campana, A.; Leonardi, F.; Casalini, S.; Kyndiah, A.; Murgia, M.; Biscarini, F. Water-gated organic field effect transistors e opportunities for biochemical sensing and extracellular signal transduction. J. Mater. Chem. B 2013, 1, 3728–3741. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.W.; White, H.S.; Wrighton, M.S. Poly(3-methylthiophene)-coated electrodes: Optical and electrical properties as a function of redox potential and amplification of electrical and chemical signals using poly(3-methylthiophene)-based microelectrochemical transistors. J. Phys. Chem. 1985, 89, 5133–5140. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, M.; Forchheimer, R.; Inganäs, O. Towards woven logic from organic electronic fibres. Nat. Mater. 2007, 6, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile organic electrochemical transistors as a platform for wearable biosensors. Sci. Rep. 2016, 6, 33637. [Google Scholar] [CrossRef] [PubMed]
- Mattana, G.; Cosseddu, P.; Fraboni, B.; Malliaras, G.G.; Hinestroza, J.P.; Bonfiglio, A. organic electronics on natural cotton fibres. Org. Electron. 2011, 12, 2033–2039. [Google Scholar] [CrossRef]
- Basiricò, L.; Cosseddu, P.; Scidà, A.; Fraboni, B.; Malliaras, G.G.; Bonfiglio, A. electrical characteristics of ink-jet printed, all-polymer electrochemical transistors. Org. Electron. 2012, 13, 244–248. [Google Scholar] [CrossRef]
- Mariani, F.; Gualandi, I.; Tessarolo, M.; Fraboni, B.; Scavetta, E. PEDOT: Dye-Based, flexible organic electrochemical transistor for highly sensitive pH monitoring. ACS Appl. Mater. Interfaces 2018, 10, 22474–22484. [Google Scholar] [CrossRef]
- Caboni, A.; Orgiu, E.; Scavetta, E.; Barbaro, M.; Bonfiglio, A. Organic-based sensor for chemical detection in aqueous solution. Appl. Phys. Lett. 2009, 95, 123304. [Google Scholar] [CrossRef]
- Ji, X.; Lau, H.Y.; Ren, X.; Peng, B.; Zhai, P.; Feng, S.-P.; Chan, P.K.L. Highly sensitive metabolite biosensor based on organic electrochemical transistor integrated with microfluidic channel and poly(n-vinyl-2-pyrrolidone)-capped platinum nanoparticles. Adv. Mater. Technol. 2016, 1, 1600042. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-J.; Lee, N.-E.; Park, J.-S.; Park, I.-J.; Kim, J.-G.; Cho, H.J. Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens. Bioelectron. 2010, 25, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yan, F.; Lin, P.; Xu, J.; Chan, H.L.W. Highly sensitive glucose biosensors based on organic electrochemical transistors using platinum gate electrodes modified with enzyme and nanomaterials. Adv. Funct. Mater. 2011, 21, 2264–2272. [Google Scholar] [CrossRef]
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; DeFranco, J.A.; Takamatsua, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2008, 18, 116–120. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Yang, Y.; Liu, K.; Du, W. Highly selective and sensitive glucose sensors based on organic electrochemical transistors using TiO2 nanotube arrays-based gate electrodes. Sens. Actuators B 2015, 208, 457–463. [Google Scholar] [CrossRef]
- Pappa, A.-M.; Curto, V.F.; Braendlein, M.; Strakosas, X.; Donahue, M.J.; Fiocchi, M.; Malliaras, G.G.; Owens, R.M. Organic transistor arrays integrated with finger-powered microfluidics for multianalyte saliva testing. Adv. Healthc. Mater. 2016, 5, 2295–2302. [Google Scholar] [CrossRef]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable sensor system for detection of lactate in sweat. Sci. Rep. 2018, 8, 15890. [Google Scholar] [CrossRef] [Green Version]
- Braendlein, M.; Pappa, A.-M.; Ferro, M.; Lopresti, A.; Acquaviva, C.; Mamessier, E.; Malliaras, G.G.; Owens, R.M. Lactate detection in tumor cell cultures using organic transistor circuits. Adv. Mater. 2017, 29, 1605744. [Google Scholar] [CrossRef]
- Strakosas, X.; Huerta, M.; Donahue, M.J.; Hama, A.; Pappa, A.-M.; Ferro, M.; Ramuz, M.; Rivnay, J.; Owens, R.M. Catalytically enhanced organic transistors for in vitro toxicology monitoring through hydrogel entrapment of enzymes. J. Appl. Pol. Sci. 2017, 134, 44483. [Google Scholar] [CrossRef]
- Mignani, A.; Scavetta, E.; Tonelli, D. Electrodeposited glucose oxidase/anionic clay for glucose biosensors design. Anal. Chim. Acta 2006, 577, 98–106. [Google Scholar] [CrossRef]
- Shan, D.; Cosnier, S.; Mousty, C. Layered double hydroxides: An attractive material for electrochemical biosensor design. Anal. Chem. 2003, 75, 3872–3879. [Google Scholar] [CrossRef] [PubMed]
- Carpani, I.; Guadagnini, L.; Tonelli, D. Lactate biosensor based on hydrotalcite-like compounds: Performances and application to serum samples. Electroanalysis 2009, 21, 2401–2409. [Google Scholar] [CrossRef]
- Volkov, A.V.; Wijeratne, K.; Mitraka, E.; Ail, U.; Zhao, D.; Tybrandt, K.; Andreasen, J.W.; Berggren, M.; Crispin, X.; Zozoulenko, I.V. Understanding the capacitance of PEDOT:PSS. Adv. Funct. Mater. 2017, 27, 1700329. [Google Scholar] [CrossRef]
- Woods, R. Chemisorption at electrodes: Hydrogen and oxygen on noble metals and their alloys. In Electroanalytical Chemistry: A Series of Advances; Bard, A.J., Ed.; Dekker: New York, NY, USA; Basel, Switzerland, 1976; Volume 9, pp. 1–162. [Google Scholar]
- Indira, L.; Kamath, P.V. Electrogeneration of base by cathodic reduction of anions: Novel one-step route to unary and Layered Double Hydroxides (LDHs). J. Mater. Chem. 1994, 4, 1487–1490. [Google Scholar] [CrossRef]
- Guadagnini, L.; Ballarin, B.; Mignani, A.; Scavetta, E.; Tonelli, D. Microscopy techniques for the characterization of modified electrodes in the development of glucose biosensors. Sens. Actuators B 2007, 126, 492–498. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Simon, D.T.; Pham, M.-C.; Noël, V.; Berggren, M. Detection of glutamate and acetylcholine with organic electrochemical transistors based on conducting polymer/platinum nanoparticle composites. Adv. Mater. 2014, 26, 5658–5664. [Google Scholar] [CrossRef]
- Cicoira, F.; Sessolo, M.; Yaghmazadeh, O.; DeFranco, J.A.; Yang, S.Y.; Malliaras, G.G. Influence of device geometry on sensor characteristics of planar organic electrochemical transistors. Adv. Mater. 2010, 22, 1012–1016. [Google Scholar] [CrossRef]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. NPJ Flex. Electron. 2018, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Mäkilä, E.; Kirveskari, P. A study of ascorbic acid in human saliva. Arch. Oral Biol. 1969, 14, 1285–1292. [Google Scholar] [CrossRef]
- Soukup, M.; Biesiada, I.; Henderson, A.; Idowu, B.; Rodeback, D.; Ridpath, L.; Bridges, E.G.; Nazar, A.M.; Grove Bridges, K. Salivary uric acid as a noninvasive biomarker of metabolic syndrome. Diabetol. Metab. Syndr. 2012, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Malon, R.S.P.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. J. Biomed. Biotechnol. 2014, 4, 962903. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.P.; Reid, R.C.; Milton, R.D.; Minteer, S.D. A self-powered amperometric lactate biosensor based on lactate oxidase immobilized in dimethylferrocene-modified LPEI. Biosens. Bioelectron. 2016, 77, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batra, B.; Narwal, V.; Pundir, C.S. An amperometric lactate biosensor based on lactate dehydrogenase immobilized onto graphene oxide nanoparticles-modified pencil graphite electrode. Eng. Life Sci. 2016, 16, 786–794. [Google Scholar] [CrossRef]






| Ag/Ach | LOD (mM) | Ig Sensitivity (mA M−1 cm−2) | Id Sensitivitylin (mA M−1 cm−2) | Gainlin | Id Sensitivitylog (mA decade−1 cm−2) | Gainlog |
|---|---|---|---|---|---|---|
| 2 | 0.15 | 0.18 ± 0.04 | 64 ± 4 | 360 | 0.10 ± 0.05 | 160 |
| 4 | 0.02 | 0.12 ± 0.02 | 43 ± 5 | 360 | 0.043 ± 0.008 | 220 |
| 8 | 0.03 | 0.036 ± 0.008 | N/E | N/E | 0.024 ± 0.005 | 120 |
| Amperometric Sensor | 0.3 | 0.18 ± 0.03* | N/A | N/A | N/A | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualandi, I.; Tessarolo, M.; Mariani, F.; Arcangeli, D.; Possanzini, L.; Tonelli, D.; Fraboni, B.; Scavetta, E. Layered Double Hydroxide-Modified Organic Electrochemical Transistor for Glucose and Lactate Biosensing. Sensors 2020, 20, 3453. https://doi.org/10.3390/s20123453
Gualandi I, Tessarolo M, Mariani F, Arcangeli D, Possanzini L, Tonelli D, Fraboni B, Scavetta E. Layered Double Hydroxide-Modified Organic Electrochemical Transistor for Glucose and Lactate Biosensing. Sensors. 2020; 20(12):3453. https://doi.org/10.3390/s20123453
Chicago/Turabian StyleGualandi, Isacco, Marta Tessarolo, Federica Mariani, Danilo Arcangeli, Luca Possanzini, Domenica Tonelli, Beatrice Fraboni, and Erika Scavetta. 2020. "Layered Double Hydroxide-Modified Organic Electrochemical Transistor for Glucose and Lactate Biosensing" Sensors 20, no. 12: 3453. https://doi.org/10.3390/s20123453
APA StyleGualandi, I., Tessarolo, M., Mariani, F., Arcangeli, D., Possanzini, L., Tonelli, D., Fraboni, B., & Scavetta, E. (2020). Layered Double Hydroxide-Modified Organic Electrochemical Transistor for Glucose and Lactate Biosensing. Sensors, 20(12), 3453. https://doi.org/10.3390/s20123453