Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instruments
2.3. Synthesis of Nitrogen-Doped Graphene
2.4. Modification of Glassy-Carbon Electrodes with Graphene-Based Materials
3. Results and Discussions
3.1. Morphological and Structural Characterization of the Nitrogen-Doped Graphene Samples
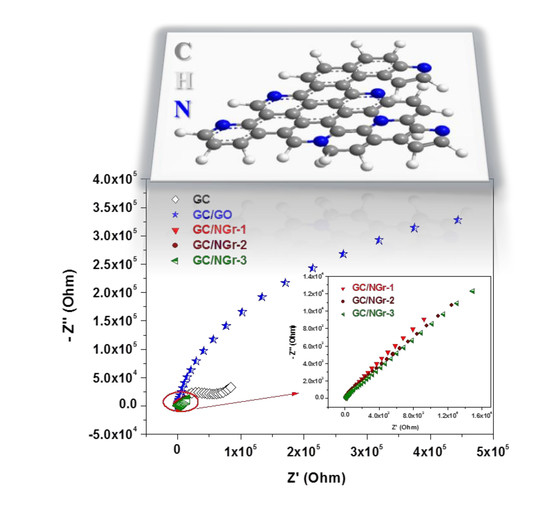
3.2. Electrochemical Studies
3.3. Electrochemical Detection of 8-OHdG with Bare and Graphene-Modified Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shams, S.S.; Zhang, R.; Zhu, J. Graphene synthesis: A Review. Mater. Sci. Pol. 2015, 33, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Cen, C.; Zhang, Y.; Chen, X.; Yang, H.; Yi, Z.; Yao, W.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. A dual-band metamaterial absorber for graphene surface plasmon resonance at terahertz frequency. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 117, 113840. [Google Scholar] [CrossRef]
- Cen, C.; Chen, Z.; Xu, D.; Jiang, L.; Chen, X.; Yi, Z.; Wu, P.; Li, G.; Yi, Y. High quality factor, high sensitivity metamaterial graphene—Perfect absorber based on critical coupling theory and impedance matching. Nanomaterials 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Damodar, D.; Kumar, S.K.; Martha, S.K.; Deshpande, A.S. Nitrogen-doped graphene-like carbon nanosheets from commercial glue: Morphology, phase evolution and Li-ion battery performance. Dalt. Trans. 2018, 47, 12218–12227. [Google Scholar] [CrossRef]
- Agnoli, S.; Favaro, M. Doping graphene with boron: A review of synthesis methods, physicochemical characterization, and emerging applications. J. Mater. Chem. A 2016, 4, 5002–5025. [Google Scholar] [CrossRef]
- Thaweesak, S.; Wang, S.; Lyu, M.; Xiao, M.; Peerakiatkhajohn, P.; Wang, L. Boron-doped graphitic carbon nitride nanosheets for enhanced visible light photocatalytic water splitting. Dalt. Trans. 2017, 46, 10714–10720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Wang, Y.; Wang, M.; Qiu, R.; Shu, Z.; Zhang, L.; Hua, Z.; Cui, F.; Wei, C.; et al. One-step synthesis of sulfur doped graphene foam for oxygen reduction reactions. Dalt. Trans. 2014, 43, 3420–3423. [Google Scholar] [CrossRef]
- Kakaei, K.; Balavandi, A. Synthesis of halogen-doped reduced graphene oxide nanosheets as highly efficient metal-free electrocatalyst for oxygen reduction reaction. J. Colloid Interface Sci. 2016, 463, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Hu, D.; Zhang, X.; Liu, D.; Wang, C. Hierarchical porous hollow FeFe(CN)6 nanospheres wrapped with I-doped graphene as anode materials for lithium-ion batteries. Dalt. Trans. 2019, 48, 4058–4066. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Bao, S.J.; Gou, Y.T.; Cai, C.J.; Ji, C.C.; Xu, M.W.; Song, J.; Wang, R. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Shan, H.; Fan, L.; Wu, C.; Li, D.; Lu, S. Superior cathode performance of nitrogen-doped graphene frameworks for lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 10643–10651. [Google Scholar] [CrossRef] [PubMed]
- Elessawy, N.A.; El Nady, J.; Wazeer, W.; Kashyout, A.B. Development of high-performance supercapacitor based on a novel controllable green synthesis for 3D nitrogen doped graphene. Sci. Rep. 2019, 9, 1129. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, Q.; Feng, X.; Pan, L.; Wu, Z.; Wu, X.; Ma, T.; Liu, J.; Pan, Y.; Song, Y.; et al. Pyridinic nitrogen-doped graphene nanoshells boost the catalytic efficiency of palladium nanoparticles for the N-allylation reaction. ChemSusChem 2019, 12, 858–865. [Google Scholar] [CrossRef]
- Oh, T.; Ryu, S.; Oh, H.; Kim, J. MnCo2O4 nanoparticles supported on nitrogen and sulfur co-doped mesoporous carbon spheres as efficient electrocatalysts for oxygen catalytic reactions. Dalt. Trans. 2019, 48, 945–953. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Wakeland, S.; Martinez, R.; Grey, J.K.; Luhrs, C.C. Production of graphene from graphite oxide using urea as expansion-reduction agent. Carbon 2010, 48, 3463–3470. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Luxa, J.; Pumera, M. Nitrogen doped graphene: Influence of precursors and conditions of the synthesis. J. Mater. Chem. C 2014, 2, 2887–2893. [Google Scholar] [CrossRef]
- Deng, D.; Pan, X.; Yu, L.; Cui, Y.; Jiang, Y.; Qi, J.; Li, W.X.; Fu, Q.; Ma, X.; Xue, Q.; et al. Toward N-doped graphene via solvothermal synthesis. Chem. Mater. 2011, 23, 1188–1193. [Google Scholar] [CrossRef]
- Shazali, S.S.; MohdZubir, M.N.; Rozali, S.; Zabri, M.Z.; Sabri, M.F.M.; Amiri, A. Facile hydrothermal method for synthesizing nitrogen-doped graphene nanoplatelets using aqueous ammonia: Dispersion, stability in solvents and thermophysical performances. Mater. Res. Express 2018, 5, 035042. [Google Scholar] [CrossRef]
- Magerusan, L.; Socaci, C.; Pogacean, F.; Rosu, M.C.; Biris, A.R.; Coros, M.; Turza, A.; Floare-Avram, V.; Katona, G.; Pruneanu, S. Enhancement of peroxidase-like activity of N-doped graphene assembled with iron-tetrapyridylporphyrin. RSC Adv. 2016, 6, 79497–79506. [Google Scholar] [CrossRef]
- Yanilmaz, A.; Tomak, A.; Akbali, B.; Bacaksiz, C.; Ozceri, E.; Ari, O.; Senger, R.T.; Selamet, Y.; Zareie, H.M. Nitrogen doping for facile and effective modification of graphene surfaces. RSC Adv. 2017, 7, 28383–28392. [Google Scholar] [CrossRef] [Green Version]
- Bundaleska, N.; Henriques, J.; Abrashev, M.; Botelho do Rego, A.M.; Ferraria, A.M.; Almeida, A.; Dias, F.M.; Valcheva, E.; Arnaudov, B.; Upadhyay, K.K.; et al. Large-scale synthesis of free-standing N-doped graphene using microwave plasma. Sci. Rep. 2018, 8, 12595. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Zhao, C.; Ge, S.; Luo, T.; Chen, J.; Liu, J.; Xi, F.; Liu, J. Gram-scale synthesis of nitrogen doped graphene quantum dots for sensitive detection of mercury ions and L-cysteine. RSC Adv. 2019, 9, 32977–32983. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhu, J.; Bi, Y.; Ren, H.; Chen, X.; Yu, H. Nitrogen-doped porous graphene-based aerogels toward efficient heavy metal ion adsorption and supercapacitor applications. Phys. Status Solidi Rapid Res. Lett. 2020, 14, 1900534. [Google Scholar] [CrossRef]
- Suhag, D.; Singh, A.; Chattopadhyay, S.; Chakrabarti, S.; Mukherjee, M. Hydrothermal synthesis of nitrogen doped graphene nanosheets from carbon nanosheets with enhanced electrocatalytic properties. RSC Adv. 2015, 5, 39705–39713. [Google Scholar] [CrossRef]
- Ren, H.; Shi, X.; Zhu, J.; Zhang, Y.; Bi, Y.; Zhang, L. Facile synthesis of N-doped graphene aerogel and its application for organic solvent adsorption. J. Mater. Sci. 2016, 51, 6419–6427. [Google Scholar] [CrossRef]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.H. Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 2010, 26, 16096–16102. [Google Scholar] [CrossRef]
- Zhang, H.; Kuila, T.; Kim, N.H.; Yu, D.S.; Lee, J.H. Simultaneous reduction, exfoliation, and nitrogen doping of graphene oxide via a hydrothermal reaction for energy storage electrode materials. Carbon 2014, 69, 66–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, G.; Gao, P.; Bi, S.; Tang, X.; Wang, D. High-performance supercapacitor of macroscopic graphene hydrogels by partial reduction and nitrogen doping of graphene oxide. Electrochim. Acta 2016, 221, 167–176. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Li, H.; Dugnani, R.; Du, Q.; UrRehman, H.; Kang, H.; Liu, H. Facile synthesis of three-dimensional lightweight nitrogen-doped graphene aerogel with excellent electromagnetic wave absorption properties. J. Mater. Sci. 2018, 53, 4067–4077. [Google Scholar] [CrossRef]
- ZangenehKamali, K.; Moradi Golsheikh, A. Green and facile approach to synthesis of well-dispersed nitrogen-doped graphene without using surfactant or stabilizer with potential application for oxygen reduction reaction. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 509, 574–582. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Wu, D.; Xia, W.; Zhao, H.; Jia, D. Hydrothermal synthesis of nitrogen-doped graphene hydrogels using amino acids with different acidities as doping agents. J. Mater. Chem. A 2014, 2, 8352–8361. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-pot hydrothermal synthesis of Nitrogen-doped graphene as high-performance anode materials for lithium ion batteries. Sci. Rep. 2016, 6, 26146. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Q.; Fu, M.; Fan, X.; Lu, H.; Wang, H.; Zhang, Y.; Wang, H. Carbon quantum dots encapsulated in super small platinum nanocrystals core-shell architecture/nitrogen doped graphene hybrid nanocomposite for electrochemical biosensing of DNA damage biomarker-8-hydroxy-2′-deoxyguanosine. Anal. Chim. Acta 2019, 1047, 9–20. [Google Scholar] [CrossRef]
- Tian, H.; Wang, L.; Sofer, Z.; Pumera, M.; Bonanni, A. Doped graphene for DNA analysis: The electrochemical signal is strongly influenced by the kind of dopant and the nucleobase structure. Sci. Rep. 2016, 6, 33046. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, X.; Fu, Z.; He, J.; Wang, C.; Wu, W.; Zhang, L. Three-dimensional macroassemblyof sandwich-like, hierarchical, porous carbon/graphene nanosheets towards ultralight, superhigh surface area, multifunctional aerogels. Chem. A Eur. J. 2016, 22, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Pogacean, F.; Socaci, C.; Pruneanu, S.; Biris, A.R.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide—A comparison study of their intrinsic peroxidase catalytic behavior. Sens. Actuators B Chem. 2015, 213, 474–483. [Google Scholar] [CrossRef]
- Magerusan, L.; Pogacean, F.; Socaci, C.; Coros, M.; Rosu, M.-C.; Pruneanu, S. Charge transfer-resistance in nitrogen-doped/undoped graphene: Its influence on the electro-catalytic reduction of H2O2. Electrochim. Acta 2016, 220, 664–671. [Google Scholar] [CrossRef]
- Coroş, M.; Pogăcean, F.; Roşu, M.-C.; Socaci, C.; Borodi, G.; Mageruşan, L.; Biriş, A.R.; Pruneanu, S. Simple and cost-effective synthesis of graphene by electrochemical exfoliation of graphite rods. RSC Adv. 2016, 6, 2651–2661. [Google Scholar] [CrossRef]
- Razeghi, M.; Pircheraghi, G. TPU/graphene nanocomposites: Effect of graphene functionality on the morphology of separated hard domains in thermoplastic polyurethane. Polymer 2018, 148, 169–180. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Caņado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size la of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Chen, H.; Wang, R.; Li, D.; Qu, J.; Dai, L. Nitrogen-doped graphene foams as metal-free counter electrodes in high-performance dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar] [CrossRef]
- Geng, D.; Yang, S.; Zhang, Y.; Yang, J.; Liu, J.; Li, R.; Sham, T.K.; Sun, X.; Ye, S.; Knights, S. Nitrogen doping effects on the structure of graphene. Appl. Surf. Sci. 2011, 257, 9193–9198. [Google Scholar] [CrossRef]
- Shang, Y.; Xu, H.; Li, M.; Zhang, G. Preparation of N-doped graphene by hydrothermal method and interpretation of N-doped mechanism. Nano 2017, 12, 1750018. [Google Scholar] [CrossRef]
- Agusu, L.; Ahmad, L.O.; Nurdin, M.; Mitsudo, S.; Kikuchi, H. Hydrothermal synthesis of reduced graphene oxide using urea as reduction agent: Excellent X-band electromagnetic absorption properties. In Proceedings of the 5th International Conference on Advanced Materials Sciences and Technology (ICAMST 2017), Makassar, Indonesia, 19–20 September 2017; Volume 367. [Google Scholar]
- Zhang, Y.; Sun, Z.; Wang, H.; Wang, Y.; Liang, M.; Xue, S. Nitrogen-doped graphene as a cathode material for dye-sensitized solar cells: Effects of hydrothermal reaction and annealing on electrocatalytic performance. RSC Adv. 2015, 5, 10430–10439. [Google Scholar] [CrossRef]
- Zeraatkar Moghaddam, A.; Ghiamati, E.; Pakar, R.; Sabouri, M.R.; Ganjali, M.R. A novel and an efficient 3-D high nitrogen doped graphene oxide adsorbent for the removal of congo red from aqueous solutions. Pollution 2019, 5, 501–514. [Google Scholar]
- Tian, C.; Sun, L.; Wang, L.; Li, M.; Shi, K.; Xie, Y.; Fu, H.; Tan, T. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar]
- Xu, X.; Zhou, Y.; Yuan, T.; Li, Y. Methanol electrocatalytic oxidation on Pt nanoparticles on nitrogen doped graphene prepared by the hydrothermal reaction of graphene oxide with urea. Electrochim. Acta 2013, 112, 587–595. [Google Scholar] [CrossRef]
- Guo, H.L.; Su, P.; Kang, X.; Ning, S.K. Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J. Mater. Chem. A 2013, 1, 2248–2255. [Google Scholar] [CrossRef]
- Chen, X.L.; Wu, N.; Gou, G.Z.; Shi, L.; Pan, S.Q.; Liu, W. Large-scale growth of nitrogen-doped via solvothermal synthesis. Appl. Mech. Mater. 2014, 670–671, 323–326. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the electrochemical area of screen-printed electrochemical sensing platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-doped graphene and its biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Nkosi, D.; Pillay, J.; Ozoemena, K.I.; Nouneh, K.; Oyama, M. Heterogeneous electron transfer kinetics and electrocatalytic behaviour of mixed self-assembled ferrocenes and SWCNT layers. Phys. Chem. Chem. Phys. 2010, 12, 604–613. [Google Scholar] [CrossRef] [Green Version]











| Sample | Reaction Time (Hours) | wt.% | |||
|---|---|---|---|---|---|
| C | N | H | O | ||
| GO | 2 | 52.84 | - | 1.53 | 5.63 |
| NGr-1 | 3 | 80.57 | 6.36 | 1.39 | 11.68 |
| NGr-2 | 8 | 80.27 | 6.37 | 1.09 | 12.27 |
| NGr-3 | 12 | 80.45 | 6.85 | 1.34 | 11.36 |
| Sample | 2θ (deg) | D (nm) | d (nm) | n | % |
|---|---|---|---|---|---|
| GO | 11.56 | 6.78 | 0.77 | 9 | 92 |
| 15.65 | 2.85 | 0.56 | 5 | 8 | |
| NGr-1 | 20.23 | 3.09 | 0.44 | 7 | 11 |
| 23.16 | 2.88 | 0.38 | 8 | 45 | |
| 25.52 | 2.53 | 0.35 | 7 | 44 | |
| NGr-2 | 16.05 | 4.02 | 0.55 | 7 | 4 |
| 19.99 | 2.35 | 0.44 | 5 | 16 | |
| 23.76 | 1.82 | 0.37 | 5 | 80 | |
| NGr-3 | 20.39 | 1.66 | 0.43 | 4 | 28 |
| 23.35 | 4.07 | 0.38 | 11 | 29 | |
| 25.47 | 2.89 | 0.35 | 8 | 43 |
| Sample | G (cm−1) | IG (a.u.) | D (cm−1) | ID (a.u.) | 2D (cm−1) | I2D (a.u.) | D+D’ (cm−1) | ID+D’ (a.u.) | ID/IG | I2D/IG | La (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GO | 1599 | 1241 | 1355 | 1203 | 2805 | 600 | 2955 | 629 | 0.97 | 0.48 | 17.11 |
| NGr-1 | 1600 | 1449 | 1356 | 1482 | 2714 | 635 | 2939 | 643 | 1.022 | 0.44 | 16.24 |
| NGr-2 | 1606 | 1212 | 1362 | 1245 | 2708 | 594 | 2940 | 607 | 1.027 | 0.49 | 16.16 |
| NGr-3 | 1600 | 1007 | 1356 | 1040 | 2702 | 548 | 2940 | 552 | 1.03 | 0.54 | 16.12 |
| Sample | Reaction Time (h) | XPS Survey (at.%) | N 1s Core-Level (%) | ||||
|---|---|---|---|---|---|---|---|
| C | O | N | Pyridinic | Pyrolic | Graphitic | ||
| GO | 2 | 70.7 | 29.3 | - | - | - | - |
| NGr-1 | 3 | 85.6 | 6.8 | 7.5 | 31.1 | 55.4 | 13.5 |
| NGr-2 | 8 | 87.2 | 6.1 | 6.6 | 38.6 | 47.6 | 13.8 |
| NGr-3 | 12 | 85.9 | 7.6 | 6.4 | 36 | 46.3 | 17.7 |
| Elements | Binding Energy (eV)/[at.%] | Assignments | |||
|---|---|---|---|---|---|
| GO | NGr-1 | NGr-2 | NGr-3 | ||
| C1s | 284.43 (31.2%) | 284.5eV (39.6%) | 284.4eV (44.4%) | 284.4eV (41.8%) | C=C |
| 285.4 (11%) | 285eV (38.9%) | 285eV (25.6%) | 285.2eV (31.4%) | C-C | |
| - | 286.6eV (13.9%) | 286.2eV (19.8%) | 286.7eV (17.6%) | C-N | |
| 287.75 (9%) | 288.6eV (4.7%) | 288.8eV (7%) | 289.9eV (6%) | C=O | |
| 289.3 (1.35%) | 290.9eV (2.9%) | 291.2eV (3.2%) | 291.9eV (3%) | O-C=O | |
| O 1s | 531.8 (4.87%) | 530.6eV (42.3%) | 530.4eV (33%) | 530.4eV (32.9%) | C=O |
| 532.72 (22.45%) | 532.1eV (31.7%) | 531.9eV (37.3%) | 531.9eV (43.3%) | -OH | |
| 533.4 (0.89) | 533.5% (25.9%) | 533.1eV (24.8%) | 533.1eV (23.7%) | O-C=O | |
| GO: Urea (Weight) | Temperature (°C) | Reaction Time (h) | Nitrogen Content (at.%) | Ref. |
|---|---|---|---|---|
| 1:3 | 190 | 12 | - | [51] |
| 1:60 | 180 | 2–18 | 7.6 | [52] |
| 1:300 | 180 | 12 | - | [53] |
| 1:300 | 180 | 12 | 11.36 | [54] |
| 1:390 | 180 | 12 | 6.05 | [55] |
| 1:30 | 160 | 3 | 6.61 | [56] |
| 1:30 | 160 | 3 | 5 | [57] |
| 1:10 | 160 | 3 | 7.5 | This work |
| 1:10 | 160 | 8 | 6.6 | |
| 1:10 | 160 | 12 | 6.4 |
| Electrode | E0′ V | ΔEp V | Ipa µA | Ipc µA | Ipa/Ipc | Qa µC | Qc µC | A cm2 | Γa pmol/cm2 |
|---|---|---|---|---|---|---|---|---|---|
| GC/NGr-1 | 0.238 | 0.076 | 3.711 | −3.554 | 1.04 | 0.755 | 0.648 | 0.05 | 156 |
| GC/NGr-2 | 0.236 | 0.079 | 3.073 | −3.090 | 0.99 | 0.656 | 0.613 | 0.043 | 157 |
| GC/NGr-3 | 0.235 | 0.081 | 2.698 | −2.714 | 0.99 | 0.609 | 0.562 | 0.036 | 175 |
| GC | 0.254 | 0.366 | 1.789 | −1.493 | 1.19 | 0.394 | 0.536 | 0.02 | 204 |
| GC/GO | 0.318 | 0.232 | 0.131 | −0.08 | 1.54 | 0.034 | 0.02 | 0.008 | 44 |
| Electrode | GC/NGr-1 | GC/NGr-2 | GC/NGr-3 | GC | GC/GO |
|---|---|---|---|---|---|
| Rct(Ohm) | 38.3 | 257 | 112 | 50.9 × 103 | 145 × 103 |
| Kapp (cm/s) | 13.9 × 10−2 | 2.39 × 10−2 | 6.55 × 10−2 | 2.6 × 10−4 | 2.1 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coros, M.; Varodi, C.; Pogacean, F.; Gal, E.; Pruneanu, S.M. Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate. Sensors 2020, 20, 1815. https://doi.org/10.3390/s20071815
Coros M, Varodi C, Pogacean F, Gal E, Pruneanu SM. Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate. Sensors. 2020; 20(7):1815. https://doi.org/10.3390/s20071815
Chicago/Turabian StyleCoros, Maria, Codruta Varodi, Florina Pogacean, Emese Gal, and Stela M. Pruneanu. 2020. "Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate" Sensors 20, no. 7: 1815. https://doi.org/10.3390/s20071815
APA StyleCoros, M., Varodi, C., Pogacean, F., Gal, E., & Pruneanu, S. M. (2020). Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate. Sensors, 20(7), 1815. https://doi.org/10.3390/s20071815