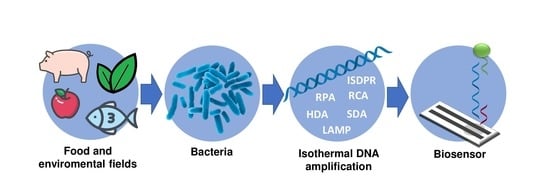
Biosensors Based on Isothermal DNA Amplification for Bacterial Detection in Food Safety and Environmental Monitoring
Abstract
:1. Introduction
2. Isothermal DNA Amplification-Based Biosensors: State of the Art
2.1. LAMP-Based Biosensors
2.2. RCA-Based Biosensors
2.3. RPA-Based Biosensors
2.4. HDA-Based Biosensors
2.5. SDA-Based Biosensors
2.6. ISDPR-Based Biosensors
3. Isothermal DNA Amplification-Based Biosensors: Key Factors
3.1. Operating Temperature
3.2. Assay Design
3.3. Type of Analyte
3.4. Detection Strategy
3.5. Applicability
3.6. Point-of-Need Testing
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 17 July 2020).
- Varadi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef] [PubMed]
- Toldrà, A.; O’Sullivan, C.; Diogène, J.; Campàs, M. Detecting harmful algal blooms with nucleic acid amplification-based biotechnological tools. Sci. Total Environ. 2020, 749, 141605. [Google Scholar] [CrossRef] [PubMed]
- Toldrà, A.; O’Sullivan, C.K.; Campàs, M. Detecting harmful algal blooms with isothermal molecular strategies. Trends Biotechnol. 2019, 37, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Ahmed, N.; Al-Madhagi, S.; Ortiz, M.; O’Sullivan, C.K.; Katakis, I. Direct electrochemical detection of enzyme labelled, isothermally amplified DNA. Anal. Biochem. 2020, 598, 6. [Google Scholar] [CrossRef] [PubMed]
- Toldrà, A.; Alcaraz, C.; Diogène, J.; O’Sullivan, C.K.; Campàs, M. Detection of Ostreopsis cf. ovata in environmental samples using an electrochemical DNA-based biosensor. Sci. Total Environ. 2019, 689, 655–661. [Google Scholar]
- Dong, Q.; Liu, Q.Y.; Guo, L.L.; Li, D.; Shang, X.D.; Li, B.L.; Du, Y. A signal-flexible gene diagnostic strategy coupling loop-mediated isothermal amplification with hybridization chain reaction. Anal. Chim. Acta 2019, 1079, 171–179. [Google Scholar] [CrossRef]
- Toldrà, A.; Furones, M.D.; O’Sullivan, C.K.; Campàs, M. Detection of isothermally amplified ostreid herpesvirus 1 DNA in Pacific oyster (Crassostrea gigas) using a miniaturised electrochemical biosensor. Talanta 2020, 207, 7. [Google Scholar] [CrossRef]
- Xie, S.B.; Tang, Y.; Tang, D.Y. Converting pyrophosphate generated during loop mediated isothermal amplification to ATP: Application to electrochemical detection of Nosema bombycis genomic DNA PTP1. Biosens. Bioelectron. 2018, 102, 518–524. [Google Scholar] [CrossRef]
- Zhao, J.M.; Gao, J.X.; Zheng, T.; Yang, Z.H.; Chai, Y.Q.; Chen, S.H.; Yuan, R.; Xu, W.J. Highly sensitive electrochemical assay for Nosema bombycis gene DNA PTP1 via conformational switch of DNA nanostructures regulated by H+ from LAMP. Biosens. Bioelectron. 2018, 106, 186–192. [Google Scholar] [CrossRef]
- de la Escosura-Muniz, A.; Baptista-Pires, L.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoci, A. Magnetic Bead/Gold Nanoparticle Double-Labeled Primers for Electrochemical Detection of Isothermal Amplified Leishmania DNA. Small 2016, 12, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.F.; Choi, G.; Nouri, R.; Guan, W.H. Loop-mediated isothermal amplification-coupled glass nanopore counting toward sensitive and specific nucleic acid testing. Nano Lett. 2019, 19, 7927–7934. [Google Scholar] [CrossRef] [PubMed]
- Nawattanapaiboon, K.; Kiatpathomchai, W.; Santanirand, P.; Vongsakulyanon, A.; Amarit, R.; Somboonkaew, A.; Sutapun, B.; Srikhirin, T. SPR-DNA array for detection of methicillin-resistant Staphylococcus aureus (MRSA) in combination with loop-mediated isothermal amplification. Biosens. Bioelectron. 2015, 74, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.P.; Lertanantawong, B.; Yin, L.S.; Ravichandran, M.; Heng, L.Y.; Surareungchai, W. Electrochemical genosensor assay using lyophilized gold nanoparticles/latex microsphere label for detection of Vibrio cholerae. Talanta 2015, 139, 167–173. [Google Scholar]
- Sun, W.; Qin, P.; Gao, H.W.; Li, G.C.; Jiao, K. Electrochemical DNA biosensor based on chitosan/nano-V2O5/MWCNTs composite film modified carbon ionic liquid electrode and its application to the LAMP product of Yersinia enterocolitica gene sequence. Biosens. Bioelectron. 2010, 25, 1264–1270. [Google Scholar] [CrossRef]
- Patterson, A.S.; Heithoff, D.M.; Ferguson, B.S.; Soh, H.T.; Mahan, M.J.; Plaxco, K.W. Microfluidic chip-based detection and intraspecies strain discrimination of Salmonella serovars derived from whole blood of septic mice. Appl. Environ. Microbiol. 2013, 79, 2302–2311. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.K.L.; Lu, H.F.; Wu, S.Y.; Kwok, H.C.; Ho, H.P.; Yu, S.; Cheung, A.K.L.; Kong, S.K. Detection of Panton-Valentine Leukocidin DNA from methicillin-resistant Staphylococcus aureus by resistive pulse sensing and loop-mediated isothermal amplification with gold nanoparticles. Anal. Chim. Acta 2013, 782, 46–53. [Google Scholar] [CrossRef]
- Li, T.C.; Zhu, F.J.; Guo, W.; Gu, H.X.; Zhao, J.; Yan, M.; Liu, S.Q. Selective capture and rapid identification of E. coli O157: H7 by carbon nanotube multilayer biosensors and microfluidic chip-based LAMP. RSC Adv. 2017, 7, 30446–30452. [Google Scholar] [CrossRef] [Green Version]
- Tsougeni, K.; Kaprou, G.; Loukas, C.M.; Papadakis, G.; Hamiot, A.; Eck, M.; Rabus, D.; Kokkoris, G.; Chatzandroulis, S.; Papadopoulos, V.; et al. Lab-on-Chip platform and protocol for rapid foodborne pathogen detection comprising on-chip cell capture, lysis, DNA amplification and surface-acoustic-wave detection. Sens. Actuator B Chem. 2020, 320, 9. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhu, X.Y.; Huang, Q.; Zheng, J.S.; Fu, W.L. Real-time monitoring of Mycobacterium genomic DNA with target-primed rolling circle amplification by a Au nanoparticle-embedded SPR biosensor. Biosens. Bioelectron. 2015, 66, 512–519. [Google Scholar] [CrossRef]
- Xiang, Y.; Deng, K.; Xia, H.; Yao, C.Y.; Chen, Q.H.; Zhang, L.Q.; Liu, Z.Y.; Fu, W.L. Isothermal detection of multiple point mutations by a surface plasmon resonance biosensor with Au nanoparticles enhanced surface-anchored rolling circle amplification. Biosens. Bioelectron. 2013, 49, 442–449. [Google Scholar] [CrossRef]
- Shi, D.C.; Huang, J.F.; Chuai, Z.R.; Chen, D.; Zhu, X.Y.; Wang, H.; Peng, J.; Wu, H.Y.; Huang, Q.; Fu, W.L. Isothermal and rapid detection of pathogenic microorganisms using a nano-rolling circle amplification-surface plasmon resonance biosensor. Biosens. Bioelectron. 2014, 62, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Du, C.L.; Ni, D.N.; Ran, Q.S.; Liu, F.; Jiang, D.N.; Pu, X.Y. A simple and sensitive electrochemical sensor for rapid detection of Clostridium tetani based on multi-walled carbon nanotubes. Anal. Methods 2016, 8, 8280–8287. [Google Scholar] [CrossRef]
- Tian, B.; Liao, X.Q.; Svedlindh, P.; Stromberg, M.; Wetterskog, E. Ferromagnetic resonance biosensor for homogeneous and volumetric detection of DNA. ACS Sens. 2018, 3, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, K.; Zhao, X.; Lin, Z.Q.; Liu, Z.Y.; Luo, S.; Zhang, Y.; Wang, Y.X.; Fu, W.L. Terahertz spectroscopy for the isothermal detection of bacterial DNA by magnetic bead-based rolling circle amplification. Analyst 2017, 142, 4661–4669. [Google Scholar] [CrossRef]
- Long, Y.; Zhou, X.M.; Xing, D. Sensitive and isothermal electrochemiluminescence gene-sensing of Listeria monocytogenes with hyperbranching rolling circle amplification technology. Biosens. Bioelectron. 2011, 26, 2897–2904. [Google Scholar] [CrossRef]
- del Río, J.S.; Svobodova, M.; Bustos, P.; Conejeros, P.; O’Sullivan, C. Electrochemical detection of Piscirickettsia salmonis genomic DNA from salmon samples using solid-phase recombinase polymerase amplification. Anal. Bioanal. Chem. 2016, 408, 8611–8620. [Google Scholar] [CrossRef]
- del Río, J.S.; Lobato, I.M.; Mayboroda, O.; Katakis, I.; O’Sullivan, C.K. Enhanced solid-phase recombinase polymerase amplification and electrochemical detection. Anal. Bioanal. Chem. 2017, 409, 3261–3269. [Google Scholar] [CrossRef]
- del Río, J.S.; Steylaerts, T.; Henry, O.Y.F.; Bienstman, P.; Stakenborg, T.; van Roy, W.; O’Sullivan, C.K. Real-time and label-free ring-resonator monitoring of solid-phase recombinase polymerase amplification. Biosens. Bioelectron. 2015, 73, 130–137. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de los Santos-Álvarez, N.; Lobo-Castañón, M.J. On-gold recombinase polymerase primer elongation for electrochemical detection of bacterial genome: Mechanism insights and influencing factors. ChemElectroChem 2019, 6, 793–800. [Google Scholar] [CrossRef]
- Lau, H.Y.; Wu, H.Q.; Wee, E.J.H.; Trau, M.; Wang, Y.L.; Botella, J.R. Specific and sensitive isothermal electrochemical biosensor for plant pathogen DNA detection with colloidal gold nanoparticles as probes. Sci. Rep. 2017, 7, 7. [Google Scholar] [CrossRef]
- Lau, H.Y.; Wang, Y.L.; Wee, E.J.H.; Botella, J.R.; Trau, M. Field demonstration of a multiplexed point-of-care diagnostic platform for plant pathogens. Anal. Chem. 2016, 88, 8074–8081. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.Y.C.; Xiao, W.; West, N.P.; Wee, E.J.H.; Wang, Y.L.; Trau, M. Rapid, single-Cell electrochemical detection of Mycobacterium tuberculosis using colloidal gold nanoparticles. Anal. Chem. 2015, 87, 10613–10618. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.Y.C.; Wee, E.J.H.; West, N.P.; Trau, M. Naked-eye colorimetric and electrochemical detection of Mycobacterium tuberculosis-toward rapid screening for active case finding. ACS Sens. 2016, 1, 173–178. [Google Scholar] [CrossRef]
- Barreda-García, S.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Solid-phase helicase dependent amplification and electrochemical detection of Salmonella on highly stable oligonucleotide-modified ITO electrodes. Chem. Commun. 2017, 53, 9721–9724. [Google Scholar] [CrossRef]
- Barreda-García, S.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Sequence-specific electrochemical detection of enzymatic amplification products of Salmonella genome on ITO electrodes improves pathogen detection to the single copy level. Sens. Actuator B Chem. 2018, 268, 438–445. [Google Scholar] [CrossRef]
- Torres-Chavolla, E.; Alocilja, E.C. Nanoparticle based DNA biosensor for tuberculosis detection using thermophilic helicase-dependent isothermal amplification. Biosens. Bioelectron. 2011, 26, 4614–4618. [Google Scholar] [CrossRef]
- Barreda-García, S.; González-Álvarez, M.J.; de-los-Santos-Álvarez, N.; Palacios-Gutiérrez, J.J.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Attomolar quantitation of Mycobacterium tuberculosis by asymmetric helicase-dependent isothermal DNA-amplification and electrochemical detection. Biosens. Bioelectron. 2015, 68, 122–128. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, Z.; Chen, H.; Tian, Y.; Zhou, N. A versatile signal-on electrochemical biosensor for Staphylococcus aureus based on triple-helix molecular switch. Sens. Actuator B Chem. 2021, 326, 128842. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Li, Y.Y.; Xie, G.M. Amplified electrochemical detection of mecA gene in methicillin-resistant Staphylococcus aureus based on target recycling amplification and isothermal strand-displacement polymerization reaction. Sens. Actuator B Chem. 2015, 221, 148–154. [Google Scholar] [CrossRef]
- Deng, H.M.; Gao, Z.Q. Bioanalytical applications of isothermal nucleic acid amplification techniques. Anal. Chim. Acta 2015, 853, 30–45. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadakis, G.; Murasova, P.; Hamiot, A.; Tsougeni, K.; Kaprou, G.; Eck, M.; Rabus, D.; Bilkova, Z.; Dupuy, B.; Jobst, G.; et al. Micro-nano-bio acoustic system for the detection of foodborne pathogens in real samples. Biosens. Bioelectron. 2018, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Grant, K.B.; Stressmann, F.; Ghigo, J.-M.; Marchal, D.; Limoges, B. Ultimate single-copy DNA detection using real-time electrochemical LAMP. ACS Sens. 2016, 1, 904–912. [Google Scholar] [CrossRef]
- Declercq, A.M.; Haesebrouck, F.; van den Broeck, W.; Bossier, P.; Decostere, A. Columnaris disease in fish: A review with emphasis on bacterium-host interactions. Vet. Res. 2013, 44, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.T.; Liu, S.C.; Huang, C.H.; Lin, C.J.; Huang, S.T. An integrated real-time electrochemical LAMP device for pathogenic bacteria detection in food. Electroanalysis 2018, 30, 2397–2404. [Google Scholar] [CrossRef]
- Jiang, D.; Xiang, G.; Liu, C.; Yu, J.; Liu, L.; Pu, X. Development of a cyclic voltammetry method for DNA electrochemical detection on microfluidic gene chip. Int. J. Electrochem. Sci. 2012, 7, 10607–10619. [Google Scholar]
- Hsieh, K.W.; Patterson, A.S.; Ferguson, B.S.; Plaxco, K.W.; Soh, H.T. Rapid, sensitive, and quantitative detection of pathogenic DNA at the point of care through microfluidic electrochemical quantitative Loop-Mediated Isothermal Amplification. Angew. Chem. Int. Edit. 2012, 51, 4896–4900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.U.; Nahar, S.; Safavieh, M.; Zourob, M. Real-time electrochemical detection of pathogen DNA using electrostatic interaction of a redox probe. Analyst 2013, 138, 907–915. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Ng, A.; Zourob, M. High-throughput real-time electrochemical monitoring of LAMP for pathogenic bacteria detection. Biosens. Bioelectron. 2014, 58, 101–106. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Tolba, M.; Zourob, M. Microfluidic electrochemical assay for rapid detection and quantification of Escherichia coli. Biosens. Bioelectron. 2012, 31, 523–528. [Google Scholar] [CrossRef]
- Kampeera, J.; Pasakon, P.; Karuwan, C.; Arunrut, N.; Sappat, A.; Sirithammajak, S.; Dechokiattawan, N.; Sumranwanich, T.; Chaivisuthangkura, P.; Ounjai, P.; et al. Point-of-care rapid detection of Vibrio parahaemolyticus in seafood using loop-mediated isothermal amplification and graphene-based screen-printed electrochemical sensor. Biosens. Bioelectron. 2019, 132, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Jaroenram, W.; Kampeera, J.; Arunrut, N.; Karuwan, C.; Sappat, A.; Khumwan, P.; Jaitrong, S.; Boonnak, K.; Prammananan, T.; Chaiprasert, A.; et al. Graphene-based electrochemical genosensor incorporated loop-mediated isothermal amplification for rapid on-site detection of Mycobacterium tuberculosis. J. Pharm. Biomed. Anal. 2020, 186, 11. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Staphylococcal (Staph) Food Poisoning. Available online: https://www.cdc.gov/foodsafety/diseases/staphylococcal.html (accessed on 17 August 2020).
- Centers for Disease Control and Prevention (CDC). Cholera—Vibrio cholera infection. Available online: https://www.cdc.gov/cholera/index.html (accessed on 17 August 2020).
- Centers for Disease Control and Prevention (CDC). Yersinia enterocolita (Yersinosis). Available online: https://www.cdc.gov/yersinia/index.html (accessed on 17 August 2020).
- Centers for Disease Control and Prevention (CDC). Salmonella Homepage. Available online: https://www.cdc.gov/salmonella/general/index.html (accessed on 17 August 2020).
- Centers for Disease Control and Prevention (CDC). E. coli (Escherichia coli). Available online: https://www.cdc.gov/ecoli/index.html (accessed on 17 August 2020).
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 11 December 2020).
- Ali, M.M.; Li, F.; Zhang, Z.Q.; Zhang, K.X.; Kang, D.K.; Ankrum, J.A.; Le, X.C.; Zhao, W.A. Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Soc. Rev. 2014, 43, 3324–3341. [Google Scholar] [CrossRef] [PubMed]
- Craw, P.; Balachandran, W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review. Lab Chip 2012, 12, 2469–2486. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.Q. Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. USA 1995, 92, 4641–4645. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/tb/default.htm (accessed on 17 August 2020).
- Melin, J.; Jarvius, J.; Larsson, C.; Soderberg, O.; Landegren, U.; Nilsson, M. Ligation-based molecular tools for lab-on-a-chip devices. New Biotech. 2008, 25, 42–48. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Available online: https://rarediseases.info.nih.gov/diseases/7123/mycobacterium-avium-complex-infections.html (accessed on 17 August 2020).
- Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/listeria/index.html (accessed on 17 August 2020).
- Piepenburg, O.; Williams, C.; Stemple, D.; Armes, N. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; von Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2020, 145, 1950–1960. [Google Scholar] [CrossRef] [Green Version]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trac-Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Vincent, M.; Xu, Y.; Kong, H.M. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795–800. [Google Scholar] [CrossRef]
- Walker, G.T.; Fraiser, M.S.; Schram, J.L.; Little, M.C.; Nadeau, J.G.; Malinowski, D.P. Strand displacement amplification—An isothermal, invitro DNA amplification technique. Nucleic Acids Res. 1992, 20, 1691–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Cheng, G.; He, P.; Fang, Y. A Review: Electrochemical Aptasensors with Various Detection Strategies. Electroanalysis 2009, 21, 1251–1259. [Google Scholar] [CrossRef]
- Guo, Q.P.; Yang, X.H.; Wang, K.M.; Tan, W.H.; Li, W.; Tang, H.X.; Li, H.M. Sensitive fluorescence detection of nucleic acids based on isothermal circular strand-displacement polymerization reaction. Nucleic Acids Res. 2009, 37, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, R.; Ihira, M.; Enomoto, Y.; Yano, H.; Maruyama, F.; Emi, N.; Asano, Y.; Yoshikawa, T. Heat denaturation increases the sensitivity of the cytomegalovirus loop-mediated isothermal amplification method. Microbiol. Immunol. 2010, 54, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Nocker, A.; Cheung, C.Y.; Camper, A.K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.M.; Spoto, G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors 2013, 3, 18–43. [Google Scholar] [CrossRef] [Green Version]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards Lab-on-a-Chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [Green Version]
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-Need DNA Testing for detection of foodborne pathogenic bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef] [Green Version]
- Erickson, D.; O’Dell, D.; Jiang, L.; Oncescu, V.; Gumus, A.; Lee, S.; Mancuso, M.; Mehta, S. Smartphone technology can be transformative to the deployment of lab-on-chip diagnostics. Lab Chip 2014, 14, 3159–3164. [Google Scholar] [CrossRef] [Green Version]






| Isothermal Amplification Method | Bacteria | Target Gene | Amplification System | Transducer Functionalisation | Detection Strategy | Detection Method | LOD | Applicability | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| LAMP | S. aureus | femB femA | In solution | Biotin-capture probes on a streptavidin-modified SPR chip | AuNP-reporter probe | Optical (SPR) | 10 copies/µL genomic DNA | Clinical samples (blood and sputum) | [14] |
| LAMP | V. cholerae | lolB | In solution | Capture probe electrodeposited on a screen-printed carbon electrode | AuNP-PSA/avidin-reporter probe | Electrochemical (DPASV) | 50 ng/µL genomic DNA | - | [15] |
| LAMP | Y. enterocolitica | gyrB | In solution | Capture probe immobilised on a chitosan-nano-V2O5-MWCNT-modified carbon paste electrode | Methylene blue intercalation into the amplicon hybridised to the capture probe | Electrochemical (DPV) | 1.76 fM synthetic DNA | Pork meat | [16] |
| LAMP | S. Typhimurium S. Choleraesuis | recF | In solution | Methylene blue-capture probes self-assembled on a gold electrode | Methylene blue-capture probes conformational change produced by target hybridisation | Electrochemical (SWV) | 350 CFU/mL | Clinical samples (blood) | [17] |
| LAMP | S. aureus | PVL toxin | In solution | Non-functionalised tuneable fluidic nanopore sensor | Biotin-primer and avidin-HRP, and AuNP-primer | Electrochemical (RPS) | 530 copies genomic DNA | - | [18] |
| LAMP | E. coli | - | In solution | Anti-E-coli polyclonal antibody immobilised on a CNT multilayer-modified ITO electrode | Monitoring of the turbidity of the magnesium pyrophosphate white precipitate produced during amplification | Optical (fiber optic sensor) | 1 CFU/mL | Spiked apple juice and milk | [19] |
| LAMP | S. Typhimurium | invA | In solution | Anti-S. Typhymurium antibody immobilised on an oxygen plasma nanotextured polymeric chip | Monitoring changes in the acoustic wave energy | Optical (SAW) | 1 CFU/25 mL | Milk | [20] |
| RCA | M. tuberculosis M. avium | - | In solution | Capture probes self-assembled on an AuNP-modified SPR chip | Amplicon cleavage and hybridisation with the capture probes | Optical (SPR) | 4.2 × 104 CFU/mL and 5 pg/µL genomic DNA for M. tuberculosis 3.7 × 104 CFU/mL and 2 pg/µL genomic DNA for M. avium | Clinical samples (sputum, urine and cerebrospinal fluid) | [21] |
| RCA | M. tuberculosis | - | Solid phase | Primer self-assembled on an SPR chip | AuNP-reporter probe | Optical (SPR) | 5 pM synthetic DNA and 8.2 pg/µL genomic DNA | Clinical samples (sputum, urine and clinical isolates) | [22] |
| RCA | E. coli E. faecalis S. dysenteriae S. pneumoniae S. epidermidis S. aureus | - | Solid phase | Biotin-primers on streptavidin-AuNPs bound to biotin-secondary antibodies immobilised on an SPR chip | AuNP-reporter probes | Optical (SPR) | 0.5 pM synthetic DNA and 0.5 pg/µL genomic DNA | Clinical samples (sputum, urine and faeces) | [23] |
| RCA | C. tetani | Tetanus toxin | In solution | Capture probe on an AuNP-MWCNT-modified glassy carbon electrode | Methylene blue-reporter probe | Electrochemical (DPV) | 1 fM synthetic DNA | Soil | [24] |
| RCA | V. cholerae | - | In solution | - | MNP-reporter probe and monitoring of the MNP aggregation | Optical (FMR) | 1 pM synthetic DNA | - | [25] |
| RCA | E. coli | - | Solid phase | Biotin-primer on streptavidin-MBs | Magnetic separation of the amplicon | Optical (THz spectroscopy) | 120 pM synthetic DNA and 50 pg/µL genomic DNA | - | [26] |
| HRCA | L. monocytogenes | hly | Solid phase | Biotin-primer on streptavidin-MBs magnetised on a platinum electrode | TBR-primer | Optical (electrochemiluminescence) | 10 aM synthetic DNA and 0.2 pg/µL genomic DNA | Milk | [27] |
| RPA | P. salmonis | - | Solid phase | Primer self-assembled on a gold electrode | Biotin-primer and streptavidin-HRP | Electrochemical (amperometry) | 50 ag/µL synthetic DNA and 3 × 102 copies/µL genomic DNA | Salmon | [28] |
| RPA | F. tularensis | - | Solid phase | Primer self-assembled on a gold electrode | Biotin-primer and streptavidin-HRP or HRP-primer | Electrochemical (amperometry) | 5.3 fM synthetic DNA and 500 fM copies genomic DNA | Hares | [29] |
| RPA | F. tularensis | - | Solid phase | Primer on an azido-modified ring resonator chip | Label-free detection | Optical (ring resonator) | 78 pM synthetic DNA | - | [30] |
| RPA | Salmonella spp. | bipA | Solid phase | Primer self-assembled on a gold electrode | FITC-primer and anti-FITC antibody-HRP | Electrochemical (amperometry) | 105 copies genomic DNA | - | [31] |
| RPA | P. syringae | - | In solution | Biotin-primer on streptavidin-MBs | AuNP-reporter probe | Electrochemical (DPV) | 214 pM genomic DNA | Arabidopsis plants | [32] |
| RPA | P. syringae B. cinerea F. oxysporum | - | In solution | Biotin-primers on streptavidin-MBs | AuNP-reporter probes complementary to the tailed-primers with different Raman reporters | Optical (SERS) | 2 copies genomic DNA | Arabidopsis and tomato plants | [33] |
| RPA | M. tuberculosis | - | In solution | Non-functionalised screen-printed carbon electrode | Biotin-dUTP amplicon and streptavidin-AuNPs | Electrochemical (DPV) | 1 CFU/mL | - | [34] |
| RPA | M. tuberculosis | - | In solution | Non-functionalised screen-printed carbon electrode | Biotin-dUTP amplicon and streptavidin-HRP | Electrochemical (amperometry) | 1 CFU/mL | - | [35] |
| HDA | Salmonella spp. | typA | Solid phase | Primer on an ITO electrode | FITC-primer and anti-FITC antibody-ALP | Electrochemical (DPV) | 10 copies synthetic DNA | - | [36] |
| HDA | Salmonella spp. | bipA | In solution | Capture probe on an ITO electrode | FITC-reporter probe and anti-FITC antibody-ALP | Electrochemical (DPV) | 50 copies synthetic DNA | - | [37] |
| HDA | M. tuberculosis | IS6110 | In solution | Capture probe on amine-MBs | AuNP-reporter probe | Electrochemical (DPV) | 10 pg/µL synthetic DNA | - | [38] |
| AHDA | M. tuberculosis | - | In solution | Biotin-primer on streptavidin-MBs magnetised on a screen-printed carbon electrode | FITC-reporter probe and anti-FITC antibody-HRP | Electrochemical (amperometry) | 0.5 aM synthetic DNA | Clinical samples (sputum, pleural fluid and urine) | [39] |
| SDA | S. aureus E. coli | - | In solution | Molecular switch fastened to G-rich capture probe on a gold electrode | Molecular switch displacement by the amplicon and formation of electroactive G-quadruplex/hemin complex | Electrochemical (DPV) | 8 CFU S. aureus/mL 22 CFU E. coli/mL | Lake water, tap water and honey | [40] |
| ISDPR | S. aureus | mecA | Solid phase | Methylene blue-hairpin probe self-assembled on a gold electrode | Methylene blue-hairpin probe conformational change produced by target hybridisation | Electrochemical (SWV) | 63 fM synthetic DNA | - | [41] |
| LAMP | RCA | RPA | HDA | SDA | ISDPR | |
|---|---|---|---|---|---|---|
| Target type | dsDNA and ssDNA, linear | dsDNA and ssDNA, circular and linear | dsDNA and ssDNA, linear | dsDNA and ssDNA, linear | dsDNA and ssDNA, linear | dsDNA and ssDNA, linear |
| Steps prior amplification | Heating at 95 °C for dsDNA targets (optional) | Heating at 95 °C for dsDNA targets; PLP ligation and exonuclease treatment at 37–95 °C for linear target | - | - | Heating at 95 °C for dsDNA targets | Heating at 95 °C for dsDNA targets |
| Amplification type | Exponential | Linear (RCA) and exponential (HRCA) | Exponential | Exponential | Exponential | Linear |
| Amplification temperature | 60–65 °C | 37–40 °C | 37–40 °C | 60–65 °C | 37 °C | 37 °C |
| Number of primers | 4–6 | 1–2 + PLP probe for linear targets | 2 | 2 | 4 | 1 + hairpin probe |
| Overall process time | ~60 min | ~150 min | 20–40 min | 60–120 min | 45–120 min | ~120 min |
| Steps after amplification | Heating at 80°C | Heating at 60°C | - | - | - | - |
| Product type | dsDNA or ssDNA, concatenated with loop configuration | dsDNA (HRCA) or ssDNA (RCA), concatenated with linear configuration | dsDNA, linear | dsDNA, linear | dsDNA or ssDNA, linear | dsDNA, linear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardo, S.; Toldrà, A.; Campàs, M. Biosensors Based on Isothermal DNA Amplification for Bacterial Detection in Food Safety and Environmental Monitoring. Sensors 2021, 21, 602. https://doi.org/10.3390/s21020602
Leonardo S, Toldrà A, Campàs M. Biosensors Based on Isothermal DNA Amplification for Bacterial Detection in Food Safety and Environmental Monitoring. Sensors. 2021; 21(2):602. https://doi.org/10.3390/s21020602
Chicago/Turabian StyleLeonardo, Sandra, Anna Toldrà, and Mònica Campàs. 2021. "Biosensors Based on Isothermal DNA Amplification for Bacterial Detection in Food Safety and Environmental Monitoring" Sensors 21, no. 2: 602. https://doi.org/10.3390/s21020602
APA StyleLeonardo, S., Toldrà, A., & Campàs, M. (2021). Biosensors Based on Isothermal DNA Amplification for Bacterial Detection in Food Safety and Environmental Monitoring. Sensors, 21(2), 602. https://doi.org/10.3390/s21020602