Development of [131I]I-EOE-TPZ and [131I]I-EOE-TPZMO: Novel Tirapazamine (TPZ)-Based Radioiodinated Pharmaceuticals for Application in Theranostic Management of Hypoxia
Abstract
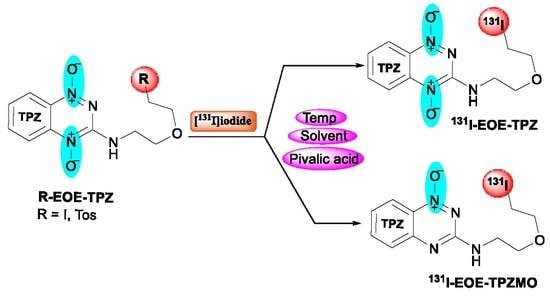
:1. Introduction
2. Results
2.1. Nucleophilic Radioiodination of P1
2.2. Isotope Exchange Radioiodination of P2
3. Discussion
4. Experimental
4.1. Materials
4.2. Methods
4.2.1. Nucleophilic Radioiodination of Tos-EOE-TPZ (Precursor P1)
4.2.2. Halogen Isotope Exchange Radioiodination of I-EOE-TPZ (Precursor P2)
4.3. Cartridge-Based Purification
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metast. Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Boss, M.K.; Dewhirst, M.W. Imaging tumor hypoxia to advance radiation oncology. Antioxid. Redox Signal. 2014, 21, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 2017, 5, 225–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.Y. Targeting tumor adaption to chronic hypoxia: Implications for drug resistance, and how it can be overcome. Int. J. Mol. Sci. 2017, 18, 1854. [Google Scholar]
- Fleming, I.N.; Manavaki, R.; Blower, P.J.; West, C.; Williams, K.J.; Harris, A.L.; Domarkas, J.; Lord, S.; Baldry, C.; Gilbert, F.J. Imaging tumor hypoxia with positron emission tomography. Br. J. Cancer 2015, 112, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.W.; Hay, M.P.M. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.; Halpern, H.J. In Vivo pO2 Imaging of tumors: Oxymetry with very low-frequency electron paramagnetic resonance. Methods Enzymol. 2015, 564, 501–527. [Google Scholar] [PubMed]
- Bernsen, M.R.; Kooiman, K.; Segbers, M.; van Leeuwen, F.W.; de Jong, M. Biomarkers in preclinical cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 579–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, C.; Lo Dico, A.; Diceglie, C.; Lucignani, G.; Ottobrini, L. Optical imaging probes in oncology. Oncotarget 2016, 7, 48753–48787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winfield, J.M.; Payne, G.S.; Weller, A.; deSouza, N.M. DCE-MRI, DW-MRI, and MRS in cancer: Challenges and advantages of implementing qualitative and quantitative multi-parametric imaging in the clinic. Top. Magn. Reson. Imaging 2016, 25, 245–254. [Google Scholar] [CrossRef]
- Cabral, P.; Cerecetto, H. Radiopharmaceuticals in tumor hypoxia imaging: A review focused on medicinal chemistry aspects. Anticancer Agents Med. Chem. 2017, 17, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bacchu, V.; Wiebe, L.I. The chemistry and radiochemistry of hypoxia-specific, radiohalogenated nitroaromatic imaging probes. Semin. Nucl. Med. 2015, 45, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, C.L.; Kumar, P.; Wiebe, L.I. Bifunctional metal-nitroimidazole complexes for hypoxia theranosis in cancer. J. Diagn. Imaging Ther. 2015, 2, 103–158. [Google Scholar] [CrossRef]
- Brown, J.M. SR 4233 (Tirapazamine): A new anticancer drug exploiting hypoxia in solid tumors. Br. J. Cancer 1993, 67, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.F.; Schofield, K. Polyazabicyclic compounds. Part II. Further derivatives of benzo-1:2:4-triazine. J. Chem. Soc. 1957. [Google Scholar] [CrossRef]
- Mason, J.C.; Tennant, G. Heterocyclic N-oxides. Part VI. Synthesis and nuclear magnetic resonance spectra of 3-aminobenzo-1,2,4-triazines and their mono- and di-N-oxides. J. Chem. Soc. B 1970, 911–916. [Google Scholar] [CrossRef]
- Zeman, E.M.; Brown, J.M.; Lemmon, M.J.; Hirst, V.K.; Lee, W.W. SR 4233: A new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1239–1242. [Google Scholar] [CrossRef]
- Zeman, E.M.; Hirst, V.K.; Lemmon, M.J.; Brown, J.M. Enhancement of radiation-induced tumor cell killing by the hypoxic cell toxin SR 4233. Radiother. Oncol. 1988, 12, 209–218. [Google Scholar] [CrossRef]
- Chopra, S.; Koolpe, G.A.; Tambo-Ong, A.A.; Matsuyama, K.N.; Ryan, K.J.; Tran, T.B.; Doppalapudi, R.S.; Riccio, E.S.; Iyer, L.V.; Green, C.E.; et al. Discovery and optimization of benzotriazine di-N-oxides targeting replicating and nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 2012, 55, 6047–6060. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, L.; Zhang, J.; Sheng, R.; Yang, B.; He, Q.; Hu, Y. Synthesis, hypoxia-selective cytotoxicity of new 3-amino-1,2,4-benzotriazine-1,4-dioxide derivatives. Eur. J. Med. Chem. 2011, 46, 919–926. [Google Scholar] [CrossRef]
- Hay, M.P.; Hicks, K.O.; Pchalek, K.; Lee, H.H.; Blaser, A.; Pruijn, F.B.; Anderson, A.F.; Shinde, S.S.; Wilson, W.R.; Denny, W.A. Tricyclic [1,2,4]Triazine 1,4-Dioxides as hypoxia selective cytotoxins. J. Med. Chem. 2008, 51, 6853–6865. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.P.; Gamage, S.A.; Kovacs, M.S.; Pruijn, F.B.; Anderson, R.F.; Patterson, A.V.; Wilson, W.R.; Brown, M.; Denny, W.A. Structure−activity relationships of 1,2,4-benzotriazine 1,4-dioxides as hypoxia-selective analogues of tirapazamine. J. Med. Chem. 2003, 46, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Elsaidi, H.R.H.; Yang, X.-H.; Ahmadi, F.; Weinfeld, M.; Wiebe, L.I.; Kumar, P. Putative electron-affinic radiosensitizers and markers of hypoxic tissue: Synthesis and preliminary in vitro biological characterization of C3-amino-substituted benzotriazine dioxides. Eur. J. Med. Chem. 2018. submitted. [Google Scholar]
- Yin, J.; Glaser, R.; Gates, K.S. On the reaction mechanism of tirapazamine reduction chemistry: Unimolecular N–OH homolysis, stepwise dehydration, or triazene ring-opening. Chem. Res. Toxicol. 2012, 25, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.F.; Shinde, S.S.; Hay, M.P.; Gamage, S.A.; Denny, W.A. Activation of 3-amino-1,2,4-benzotriazine 1,4-dioxide antitumor agents to oxidizing species following their one-electron reduction. J. Am. Chem. Soc. 2003, 125, 748–756. [Google Scholar] [CrossRef]
- Siim, B.G.; Pruijn, F.B.; Sturman, J.R.; Hogg, A.; Hay, M.P.; Brown, J.M.; Wilson, W.R. Selective potentiation of the hypoxic cytotoxicity of tirapazamine by its 1-N-oxide metabolite SR 4317. Cancer Res. 2004, 64, 736–742. [Google Scholar] [CrossRef]
- Yoo, B.W.; Park, M.C. Mild and efficient deoxygenation of amine-N-oxides with MoCl5/NaI system. Synth. Commun. 2008, 38, 1646–1650. [Google Scholar] [CrossRef]
- Revuelta, J.; Cicchi, S.; Brandi, A. Samarium(II) iodide reduction of isoxazolidines. Tetrahedron Lett. 2004, 45, 8375–8377. [Google Scholar] [CrossRef]
- Singh, D.; Singh, V.; Rai, B.P. An efficient method for the reduction of cephalosporin sulfoxide. Asian J. Chem. 2007, 19, 5787–5789. [Google Scholar]
- Das, A.K.; Srivastav, M.; Layek, R.K.; Uddin, M.E.; Jung, D.; Kim, N.H.; Lee, J.H. Iodide-mediated room temperature reduction of graphene oxide: A rapid chemical route for the synthesis of a bifunctional electrocatalyst. J. Mater. Chem. A 2014, 2, 1332–1340. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, R. Some deoxygenation and reduction reactions with samarium diiodide. Synth. Commun. 1987, 17, 329–332. [Google Scholar] [CrossRef]
- Weichert, J.P.; Van Dort, M.E.; Groziak, M.P.; Counsell, R.E. Radioiodination via isotope exchange in pivalic acid. Int. J. Rad. Appl. Instrum. A 1986, 37, 907–913. [Google Scholar] [CrossRef]







| Solvent | Temp (°C) | Time (min) | [131I]I-EOE-TPZ % of Total [131I] | [131I]I-EOE-TPZMO % of Total [131I] |
|---|---|---|---|---|
| ACN | 22 | 30 and 60 | 0, 0 | 0, 0 |
| ACN | 60 | 30 and 60 | 0, 0 | 17.2, 31.2 |
| ACN | 80 | 60 | 48.5 | 20.7 |
| ACN | 100 | 60 | 10.1 | 50.2 |
| DMF | 22 | 30 and 90 | 0, 0 | 0, 0 |
| DMF DMF DMF | 60 80 100 | 30 and 60 60 60 | 0, 0 24.6 7.8 | 0, 0 |
| 16.7 | ||||
| 34.2 |
| Solvent | Temp (°C) | Time (min) | Pivalic Acid (mg) | [131I]I-EOE-TPZ % of Total [131I] | [131I]I-EOE-TPZMO % of Total [131I] |
|---|---|---|---|---|---|
| DMF | 22 | 30, 60 | 0 | 0, 18 | 72.7, 54.3 |
| ACN | 22 | 30, 60 | 0 | 0, 0 | 34.7, 7.5 |
| ACN | 80 | 30 | 0 | 89 | 6.9 |
| ACN | 50 | 30, 60 | 3.5 | 0, 7.7 | 92.4, 66 |
| ACN | 22 | 30, 60, 90 | 3.5 | 3.2, 3.5, 3 | 54, 50, 48.7 |
| EtOH/ACN | 22 | 30, 60, 90 | 3.5 | 0, 0, 0 | 43, 42.4, 42.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaidi, H.; Ahmadi, F.; Wiebe, L.I.; Kumar, P. Development of [131I]I-EOE-TPZ and [131I]I-EOE-TPZMO: Novel Tirapazamine (TPZ)-Based Radioiodinated Pharmaceuticals for Application in Theranostic Management of Hypoxia. Pharmaceuticals 2019, 12, 3. https://doi.org/10.3390/ph12010003
Elsaidi H, Ahmadi F, Wiebe LI, Kumar P. Development of [131I]I-EOE-TPZ and [131I]I-EOE-TPZMO: Novel Tirapazamine (TPZ)-Based Radioiodinated Pharmaceuticals for Application in Theranostic Management of Hypoxia. Pharmaceuticals. 2019; 12(1):3. https://doi.org/10.3390/ph12010003
Chicago/Turabian StyleElsaidi, Hassan, Fatemeh Ahmadi, Leonard I. Wiebe, and Piyush Kumar. 2019. "Development of [131I]I-EOE-TPZ and [131I]I-EOE-TPZMO: Novel Tirapazamine (TPZ)-Based Radioiodinated Pharmaceuticals for Application in Theranostic Management of Hypoxia" Pharmaceuticals 12, no. 1: 3. https://doi.org/10.3390/ph12010003
APA StyleElsaidi, H., Ahmadi, F., Wiebe, L. I., & Kumar, P. (2019). Development of [131I]I-EOE-TPZ and [131I]I-EOE-TPZMO: Novel Tirapazamine (TPZ)-Based Radioiodinated Pharmaceuticals for Application in Theranostic Management of Hypoxia. Pharmaceuticals, 12(1), 3. https://doi.org/10.3390/ph12010003