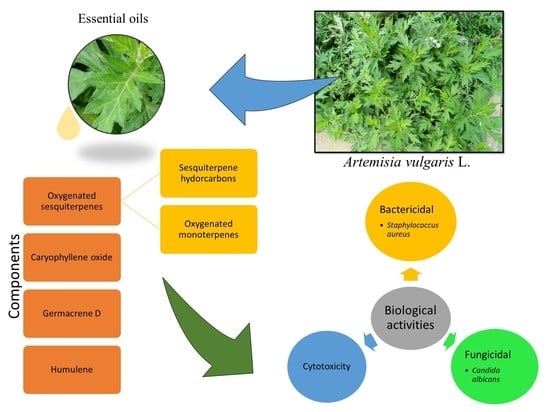
Chemical Profile and Biological Activities of Essential Oil from Artemisia vulgaris L. Cultivated in Brazil
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Botanical Identification
2.2. Extraction of Essential Oils
2.3. Identification of Compounds Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.4. Identification of Compounds
2.5. Microorganisms and Growth Conditions
2.6. Determination of Antimicrobial and Anti-Fungal Activities in Essential Oils
2.7. Parasitological Procedures
2.8. Egg Hatching Assay (EHA)
2.9. Larval Exsheathment Inhibition Assay (LEIA)
2.10. Larval Migration Inhibition Assay (LMIA)
3. Results and Discussion
3.1. Chemical Composition of Essential Oil Extracted from Brazilian A. vulgaris Leaves
3.2. Anti-Microbial Activities Identification
3.3. Anthelmintic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rauha, J.P.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Kahkonen, M. Antimicrobial effects of Finnish plant extracts containing flavoniods and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Avancini, C.A.M.; Wiest, J.M.; Mundstock, E. Atividade bacteriostática e bactericida do decocto de Baccharis trimera (Less.) D.C., Compositae, carqueja, como desinfetante ou anti-séptico. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2000, 52, 230–234. [Google Scholar] [CrossRef]
- Júnior, A.A.S.; Vizzotto, V.J.; Giorgi, E.; Macedo, S.G.; Marques, L.F. Plantas Medicinais, Caracterizacao E Cultivo; Epagri: Florianópolis, SC, Brazil, 1994. [Google Scholar]
- Konning, G.H.; Agyare, C.; Ennison, B. Antimicrobial activity of some medicinal plants from Ghana. Fitoterapia 2004, 75, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Boyayan, M. Oguaco, planta nativa da mata Atlântica, tem mais propriedades terapêuticas do que se supunha. Revista Pesquisa Fapesp 2002, 74, 48–49. [Google Scholar]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz. 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [Green Version]
- Tassou, C.; Koutsoumanis, K.; Nychas, G.J.E. Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food Res. Int. 2000, 33, 273–280. [Google Scholar] [CrossRef]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Solid- and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Di Stasi, L.C. Plantas Medicinais: Arte E Ciência: Um Guia De Estudo Interdisciplinar; Editora da Universidade Estadual Paulista: São Paulo, Brazil, 1996. [Google Scholar]
- Ahmad, I.; Beg, Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.; Lortheeranuwat, A.; Jeeju, W.; Sririrak, T.; Phongpaichit, S.; Supawita, T. Effective medicinal plants against enterohaemorrhagic Escherichia coli O157:H7. J. Ethnopharmacol. 2004, 94, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Malik, S.; Yousefzadi, M.; Makhzoum, A.; Tremouillaux-Guiller, J.; Bonfill, M. Erratum to: Taxol from Corylus avellana: Paving the way for a new source of this anti-cancer drug. Plant Cell Tissue Organ Cult. 2017, 129, 1–17. [Google Scholar] [CrossRef]
- Malik, S.; Bíba, O.; Grúz, J.; Arroo, R.R.J.; Strnad, M. Biotechnological approaches for producing aryltetralin lignans from Linum species. Phytochem. Rev. 2014, 13, 893–913. [Google Scholar] [CrossRef]
- Malik, S.; Bhushan, S.; Sharma, M.; Ahuja, P.S. Biotechnological approaches to the production of shikonins: A critical review with recent updates. Crit. Rev. Biotechnol. 2016, 36, 327–340. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Malik, S.; Costa, F.; Yousefzadi, M.; Mirjalili, M.H.; Arroo, R.; Bhambra, A.S.; Strnad, M.; Bonfill, M.; Fett-Neto, A.G. Specialized plant metabolism characteristics and impact on target molecule biotechnological production. Mol. Biotechnol. 2018, 60, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, L.S.S.; Luz, T.R.S.A.; Mesquita, J.W.C.; Coutinho, D.F.; Amaral, F.M.M.; Ribeiro, M.N.S.; Malik, S. Exploring the anticancer properties of essential oils from family Lamiaceae. Food Rev. Int. 2018, 35, 105–131. [Google Scholar] [CrossRef]
- Hussein, H.A.S.A.A.; Hussein, M.S.; Tkachenko, K.G.; Nkomo, M.; Mudau, F.N. Essential oil composition of artemisia vulgaris grown in Egypt. Int. J. Pharm. Pharm. Sci. 2016, 8, 120–123. [Google Scholar]
- Mahmood, T.; Hassan, N.; Nazar, N.; Naveed, I. Phylogenetic analysis of different Artemisia species based on chloroplast gene rps11. Arch. Biol. Sci. 2011, 63, 661–665. [Google Scholar] [CrossRef]
- Negahban, M.; Moharramipour, S.; Sefidkon, F. Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J. Stored Prod. Res. 2007, 43, 123–128. [Google Scholar] [CrossRef]
- Judžentien, A.; Buzelyte, J. Chemical composition of essential oils of Artemisia vulgaris L. (mugwort) from North Lithuania. Chemija 2006, 17, 12–15. [Google Scholar]
- Adams, J.D.; Garcia, C.; Garg, G. Mugwort (Artemisia vulgaris, Artemisia douglasiana, Artemisia argyi) in the Treatment of menopause, premenstrual syndrome, dysmenorrhea and attention deficit hyperactivity disorder. Chin. Med. 2012, 3, 116–123. [Google Scholar] [CrossRef]
- Malinowski, L.R.L.; Rosa, E.A.R.; Picheth, C.M.T.F.; Campelo, P.M.S. Atividade antimicrobiana dos extratos aquoso e hidroalcoólico de folhas de Artemisia vulgaris. Rev. Bras. Farm. 2007, 88, 63–66. [Google Scholar]
- Rajaram, A.S.R.S.K.; Reddy, C.N.K.S.P.; Sibyala, V.S. Antifertility activity of Artemisia vulgaris leaves on female Wistar rats. Chin. J. Nat. Med. 2014, 3, 4. [Google Scholar]
- Tigno, X.T.; de Guzman, F.; Flora, A.M.; Theresa, V. Phytochemical analysis and hemodynamic actions of Artemisia vulgaris L. Clin. Hemorheol. Microcirc. 2000, 23, 167–175. [Google Scholar]
- Abiri, R.; Silva, A.L.M.; Mesquita, L.S.S.; Mesquita, J.W.C.; Atabaki, N.; Almeida, E.B.; Shaharuddin, N.A.; Malik, S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018, 109, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Mucciarelli, M.; Caramiello, R.; Maffei, M.; Chialva, F. Essential oils from some Artemisia species growing spontaneously in North-West Italy. Flavour Fragr. J. 1995, 10, 25–32. [Google Scholar] [CrossRef]
- Nano, G.M.; Bicchi, C.; Frattini, C.; Gallino, M. On the composition of some oils from Artemisia vulgaris. Planta Med. 1976, 30, 211–215. [Google Scholar] [CrossRef]
- Misra, L.N.; Singh, S.P. α-Thujone, the major component of the essential oil from Artemisia vulgaris growing wild in Nilgiri hills. J. Nat. Prod. 1986, 49, 941. [Google Scholar] [CrossRef]
- Benabdellah, M.; Benkaddour, M.; Hammouti, B.; Bendahhou, M.; Aouniti, A. Inhibition of steel corrosion in 2M H 3 PO 4 by Artemisia oil. Appl. Surf. Sci. 2006, 252, 6212–6217. [Google Scholar] [CrossRef]
- Näf-Müller, R.; Pickenhagen, W.; Willhalm, B. New irregular monoterpenes in Artemisia vulgaris. Helv. Chim. Acta 1981, 64, 1424–1430. [Google Scholar]
- Alizadeh, M.; Aghaei, M.; Sharifian, I.; Saadatian, M. Chemical composition of essential oil of Artemisia vulgaris from West Azerbaijan, Iran. Electron. J. Environ. Agric. Food Chem. 2012, 11, 493–496. [Google Scholar]
- Pino, J.A.; Rosado, A.; Fuentes, V. Composition of the essential oil of Artemisia vulgaris L. herb from Cuba. J. Essent. Oil Res. 1999, 11, 477–478. [Google Scholar] [CrossRef]
- Schmidt, A.; Kochanowski, K.; Vedelaar, S.; Ahrné, E.; Volkmer, B.; Callipo, L.; Heinemann, M. The quantitative and condition-dependent Escherichia coli proteome. Nat. Biotechnol. 2016, 34, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A.; Vinces, M.D. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 2005, 7, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Glory, A.; van Oostende, C.T.; Geitmann, A.; Bachewich, C. Depletion of the mitotic kinase Cdc5p in Candida albicans results in the formation of elongated buds that switch to the hyphal fate over time in a Ume6p and Hgc1p-dependent manner. Fungal Genet. Biol. 2017, 107, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.A.; Chilton, N.B.; Gasser, R.B. Differentiation of Haemonchus placei from H. contortus (Nematoda: Trichostrongylidae) by the ribosomal DNA second internal transcribed spacer. Int. J. Parasitol. 1995, 25, 483–488. [Google Scholar] [CrossRef]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.M.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- Bahuaud, D.; De Montellano, C.M.O.; Chauveau, S.; Prevot, F.; Torres-Acosta, F.; Fouraste, I.; Hoste, H. Effects of four tanniferous plant extracts on the in vitro exsheathment of third-stage larvae of parasitic nematodes. Parasitology 2006, 132, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Galeotti, N.; Mannelli, L.D.C.; Mazzanti, G.; Bartolini, A. Local anaesthetic activity of β-caryophyllene. Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Ormeño, E.; Baldy, V.; Ballini, C.; Fernandez, C. Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: effect of soil nutrients. J. Chem. Ecol. 2008, 34, 1219. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Nat. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Kil, B.S.; Han, D.M.; Lee, C.H.; Kim, Y.S.; Yun, K.Y.; Yoo, H.G. Allelopathic effects of Artemisia lavandulaefolia. Korean J. Ecol. 2000, 23, 149–155. [Google Scholar]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Antioxidant polyphenols from tart cherries (Prunus cerasus). J. Agric. Food Chem. 1999, 47, 840–844. [Google Scholar] [CrossRef]
- Rivero-Cruz, B.; Rivero-Cruz, I.; Rodríguez, J.M.; Cerda-García-Rojas, C.M.; Mata, R. Qualitative and quantitative analysis of the active components of the essential oil from brickellia v eronicaefolia by nuclear magnetic resonance spectroscopy. J. Nat. Prod. 2006, 69, 1172–1176. [Google Scholar] [CrossRef]
- Li, F.Q.; Yang, S.P.; Chen, Y.; Lao, S.C.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2005, 39, 552–558. [Google Scholar]
- Bülow, N.; König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Scorciapino, A. Chemical composition of the essential oil and supercritical CO2 extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005, 53, 7939–7943. [Google Scholar] [CrossRef] [PubMed]
- Noge, K.; Becerra, J.X. Germacrene D, a common sesquiterpene in the genus Bursera (Burseraceae). Molecules 2009, 14, 5289–5297. [Google Scholar] [CrossRef]
- Katsiotis, S.T.; Langezaal, C.R.; Scheffer, J.J.C. Analysis of the volatile compounds from cones of ten Humulus lupulus cultivars. Planta Med. 1989, 55, 634. [Google Scholar] [CrossRef]
- El Hadri, A.; del Rio, M.A.G.; Sanz, J.; Coloma, A.G.; Idaomar, M.; Ozonas, B.R.; Reus, M.I.S. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An. Real Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharm. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharm. 2007, 59, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Peng, S.L.; Zeng, R.S.; Ding, L.W.; Xu, Z.F. Cloning, expression and wounding induction of β-caryophyllene synthase gene from Mikania micrantha HBK and allelopathic potential of β-caryophyllene. Allelopathy J. 2009, 24, 35–44. [Google Scholar]
- Lutz, D.L.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Iqbal, Z.; Lateef, M.; Ashraf, M.; Jabbar, A. Anthelmintic activity of Artemisia brevifolia in sheep. J. Ethnopharmacol. 2004, 93, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.A.; Chishti, M.Z.; Ahmad, F.; Shawl, A.S. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet. Parasitol. 2009, 160, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Caner, A.; Döşkaya, M.; Deǧirmenci, A.; Can, H.; Baykan, Ş.; Üner, A.; Gürüz, Y. Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella spiralis) in rats. Exp. Parasitol. 2008, 119, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.M.; Ferreira, J.F.S.; Lindsay, D.S.; Zajac, A.M. Effects of artemisinin and Artemisia extracts on Haemonchus contortus in gerbils (Meriones unguiculatus). Vet. Parasitol. 2011, 175, 103–108. [Google Scholar] [CrossRef]
- Zhu, L.; Dai, J.L.; Yang, L.; Qiu, J. In vitro ovicidal and larvicidal activity of the essential oil of Artemisia lancea against Haemonchus contortus (Strongylida). Vet. Parasitol. 2013, 195, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, S.C.; Magalhães, L.G.; de Melo, N.I.; Wakabayashi, K.A.L.; de P. Aguiar, G.; de P. Aguiar, D.; Tavares, D.C. Chemical composition and in vitro schistosomicidal activity of the essential oil of Plectranthus neochilus grown in Southeast Brazil. Chem. Biodivers. 2011, 8, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- De Melo, N.I.; Magalhaes, L.G.; De Carvalho, C.E.; Wakabayashi, K.A.L.; De P. Aguiar, G.; Ramos, R.C.; Groppo, M. Schistosomicidal activity of the essential oil of Ageratum conyzoides L. (Asteraceae) against adult Schistosoma mansoni worms. Molecules 2011, 16, 762–773. [Google Scholar] [CrossRef]
- Olounladé, P.A.; Azando, E.V.B.; Hounzangbé-Adoté, M.S.; Ha, T.B.T.; Leroy, E.; Moulis, C.; Valentin, A. In vitro anthelmintic activity of the essential oils of Zanthoxylum zanthoxyloides and Newbouldia laevis against Strongyloides ratti. Parasitol. Res. 2012, 110, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.O.D.; Moorkoth, S.; Azeez, S.; Eapen, S.J. Virtual screening and in vitro assay of potential drug like inhibitors from spices against Glutathione-S-Transferase of Meloidogyne incognita. Bioinformation 2012, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.H.; Bai, C.Q.; Liu, Q.Z.; Du, S.S.; Liu, Z.L. Nematicidal activity of the essential oil of Rhododendron anthopogonoides aerial parts and its constituent compounds against Meloidogyne incognita. Z. Naturforsch. C 2013, 68, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Jackson, F.; Hoste, H. In vitro methods for the primary screening of plant products for direct activity against ruminant gastrointestinal nematodes. In Vitro Screening of Plant Resources for Extra-Nutritional Attributes in Ruminants: Nuclear and Related Methodologies; Springer: Dordrecht, The Netherlands, 2010; pp. 25–45. [Google Scholar]
| No. | Compound | RI * | % |
|---|---|---|---|
| 1 | Borneol | 1173 | 6.80 |
| 2 | Bornyl acetate | 1287 | 1.46 |
| 3 | Lavandulyl acetate | 1298 | 2.83 |
| 4 | Caryophyllene | 1420 | 37.45 |
| 5 | Humulene | 1455 | 13.66 |
| 6 | Germacrene D | 1482 | 16.17 |
| 7 | α-Farnesene | 1510 | 3.11 |
| 8 | Δ-Cadinene | 1524 | 1.23 |
| 9 | Epiglobulol | 1530 | 0.58 |
| 10 | Germacrene B | 1558 | 1.39 |
| 11 | Nerolidol, acetate | 1570 | 0.49 |
| 12 | Germacren-d-4-ol | 1576 | 0.93 |
| 13 | Caryophyllene oxide | 1583 | 5.67 |
| 14 | Viridiflorol | 1592 | 0.48 |
| 15 | Isoaromadendrene epoxide | 1606 | 2.17 |
| 16 | Longipinocarveol, trans- | 1634 | 0.65 |
| 17 | α-Cadinol | 1655 | 1.99 |
| 18 | Phytol | 2112 | 2.94 |
| - | Microorganism | Positive Control | CC | ||
|---|---|---|---|---|---|
| Microorganism | MIC | MBC/MFC | MIC | MBC/MFC | |
| E. coli | 15.6 | 15.6 | 0.156 µg/mL | - | + |
| S. aureus | 62.4 | 125 | 0.078 µg/mL | - | + |
| C. albicans | 31.2 | 125 | 20 IU/mL | 20 IU/mL | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.; de Mesquita, L.S.S.; Silva, C.R.; de Mesquita, J.W.C.; de Sá Rocha, E.; Bose, J.; Abiri, R.; de Maria Silva Figueiredo, P.; Costa-Júnior, L.M. Chemical Profile and Biological Activities of Essential Oil from Artemisia vulgaris L. Cultivated in Brazil. Pharmaceuticals 2019, 12, 49. https://doi.org/10.3390/ph12020049
Malik S, de Mesquita LSS, Silva CR, de Mesquita JWC, de Sá Rocha E, Bose J, Abiri R, de Maria Silva Figueiredo P, Costa-Júnior LM. Chemical Profile and Biological Activities of Essential Oil from Artemisia vulgaris L. Cultivated in Brazil. Pharmaceuticals. 2019; 12(2):49. https://doi.org/10.3390/ph12020049
Chicago/Turabian StyleMalik, Sonia, Ludmilla Santos Silva de Mesquita, Carolina Rocha Silva, José Wilson Carvalho de Mesquita, Emmeline de Sá Rocha, Jayakumar Bose, Rambod Abiri, Patricia de Maria Silva Figueiredo, and Livio M. Costa-Júnior. 2019. "Chemical Profile and Biological Activities of Essential Oil from Artemisia vulgaris L. Cultivated in Brazil" Pharmaceuticals 12, no. 2: 49. https://doi.org/10.3390/ph12020049
APA StyleMalik, S., de Mesquita, L. S. S., Silva, C. R., de Mesquita, J. W. C., de Sá Rocha, E., Bose, J., Abiri, R., de Maria Silva Figueiredo, P., & Costa-Júnior, L. M. (2019). Chemical Profile and Biological Activities of Essential Oil from Artemisia vulgaris L. Cultivated in Brazil. Pharmaceuticals, 12(2), 49. https://doi.org/10.3390/ph12020049