Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Results
2.1. HELAp Analysis (UPLC-MS and HPLC-DAD)
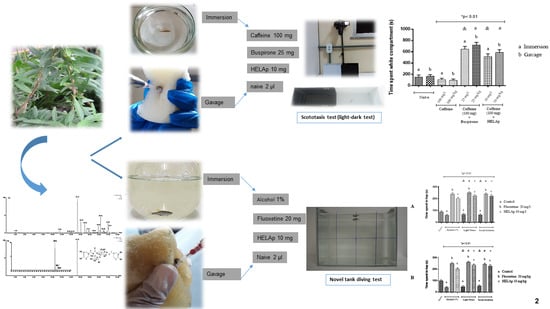
2.2. Scototaxis Test (Light–Dark Test)
2.3. Novel Tank Diving Test (NTDT)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. HELAp UPLC-MS Analysis
4.3. Acteoside Quantification Through HPLC-DAD
4.4. Animals
4.5. Anxiety Evaluation in Zebrafish
4.5.1. Drugs, Reagents, and Treatments
4.5.2. Scototaxis Test (Light–Dark Test)
4.6. Antidepressant Evaluation in Zebrafish
4.6.1. Drugs, Reagents, and Treatments
4.6.2. Developmental Social Isolation (DSI)
4.6.3. Unpredictable Chronic Mild Stress (UCMS)
4.6.4. Novel Tank Diving Test (NTDT)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; pp. 1–24. [Google Scholar]
- Muller, J.L.; Trentini, C.M.; Zanini, A.M.; Lopes, F.M. Transtorno de Ansiedade Social: Um estudo de caso. Contextos Clínicos 2015, 8, 67–78. [Google Scholar] [CrossRef]
- McNaughton, N.; Corr, P.J. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004, 28, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Reddy, B.R.; Sudhakar, S.R.; Saxena, S.; Das, T.; Meghah, V.; Brahmendra Swamy, C.V.; Kumar, A.; Idris, M.M. Chronic Unpredictable Stress (CUS)-Induced Anxiety and Related Mood Disorders in a Zebrafish Model: Altered Brain Proteome Profile Implicates Mitochondrial Dysfunction. PLoS ONE 2013, 8, e63302. [Google Scholar] [CrossRef] [PubMed]
- Filipov, A. Medicinal plants of the Pilagá of Central Chaco. J. Ethnopharmacol. 1994, 44, 181–193. [Google Scholar] [CrossRef]
- Del Vitto, L.; Petenatti, E.M.; Petenatti, M.E. Recursos herbolarios de San Luis (Argentina), Primera parte: plantas nativas. Multequina 1997, 6, 49–66. [Google Scholar]
- Hellión-Ibarrola, M.C.; Ibarrola, D.A.; Montalbetti, Y.; Kennedy, M.L.; Heinichen, O.; Campuzano, M.; Tortoriello, J.; Fernández, S.; Wasowski, C.; Marder, M.; et al. The anxiolytic-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. J. Ethnopharmacol. 2006, 105, 400–408. [Google Scholar] [CrossRef]
- Hellión-Ibarrola, M.C.; Ibarrola, D.A.; Montalbetti, Y.; Kennedy, M.L.; Heinichen, O.; Campuzano, M.; Ferro, E.A.; Alvarenga, N.; Tortoriello, J.; De Lima, T.C.M.; et al. The antidepressant-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. Phytomedicine 2008, 15, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Díaz-Véliz, G.; Millán, R.; Lungenstrass, H.; Quirós, S.; Coto-Morales, T.; Hellión-Ibarrola, M.C. Anxiolytic and antidepressant-like effects of the hydroalcoholic extract from Aloysia polystachya in rats. Pharmacol. Biochem. Behav. 2005, 82, 373–378. [Google Scholar] [CrossRef]
- Marchetti, L.; Pellati, F.; Graziosi, R.; Brighenti, V.; Pinetti, D.; Bertelli, D. Identification and determination of bioactive phenylpropanoid glycosides of Aloysia polystachya (Griseb. et Moldenke) by HPLC-MS. J. Pharm. Biomed. Anal. 2019, 166, 364–370. [Google Scholar] [CrossRef]
- Pereira, A.; Guimarães, C.; Pereira, S.; Crevelin, E.; Pinto, G.; Morel, L.; Bertoni, B.; França, S.; Taleb-Contini, S. Isolation and Identification of Phenylethanoid Glycosides from Aloysia polystachya and Its Activity as Inhibitors of Monoamine Oxidase-A. Planta Med. Int. Open 2019, 6, e1–e6. [Google Scholar] [CrossRef] [Green Version]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Friščić, M.; Bucar, F.; Hazler Pilepić, K. LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom. 2016, 51, 1211–1236. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Woo, E.R.; Choi, C.Y.; Shin, D.W.; Lee, D.G.; You, H.J.; Jeong, H.G. Protective Effect of Acteoside on Carbon Tetrachloride-Induced Hepatotoxicity. Life Sci. 2004, 74, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.Q.; Zhang, J.R.; Pu, X.P.; Ma, J.; Li, C.L. Protective effect of verbascoside on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Eur. J. Pharmacol. 2002, 451, 119–124. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Weng, X.-J.; Chen, L.-L.; Li, X. Effect of Cistanche tubulosa (Scheuk) Whight acteoside on telomerase activity and immunity of aging mice. Chin. J. Pharmacol. Toxicol. 2008, 22, 270–273. [Google Scholar] [CrossRef]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The synthenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Barros, T.P.; Alderton, W.K.; Reynolds, H.M.; Roach, A.G.; Berghmans, S. Zebrafish: An emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br. J. Pharmacol. 2008, 154, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Gerlai, R. High-throughput behavioral screens: The first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules 2010, 15, 2609–2622. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Fernandes, Y.; Pereira, T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav. Brain Res. 2009, 201, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D. Zebrafish assessment of cognitive improvement and anxiolysis: Filling the gap between in vitro and rodent models for drug development. Rev. Neurosci. 2011, 22, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Piato, A.L.; Capiotti, K.M.; Tamborski, A.R.; Oses, J.P.; Barcellos, L.J.G.; Bogo, M.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. The use of the zebrafish model in stress research. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Kaluyeva, A.A.; Poudel, M.K.; Nguyen, M.; Song, C.; Kalueff, A.V. Building Zebrafish Neurobehavioral Phenomics: Effects of Common Environmental Factors on Anxiety and Locomotor Activity. Zebrafish 2015, 12, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.; Maximino, C.; Brito, T.M.; Herculano, A.M.; Gouveia, A., Jr.; Morato, S.; Cachat, J.M.; Gaikwad, S.; Elegante, M.F.; Hart, P.C.; et al. Neurophenotyping of Adult Zebrafish Using the Light–dark Box Paradigm. In Zebrafish Neurobehavioral Protocols; Neuromethods 51; Human Press: Heidelberg, Germany, 2011; pp. 157–167. [Google Scholar] [CrossRef]
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; Sampaio, T.I.S.; Ferreira, I.M.; Lima, E.S.; Silva, M.J.A.; Fernandes, C.P.; Oliveira, A.E.F.M.; Conceição, E.C.; et al. Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: In vitro and in zebrafish studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; Keita, H.; Santana, G.R.; Souza, G.C.; Santos, I.V.F.; Amado, J.R.R.; Kourouma, A.; Prada, A.L.; Carvalho, H.O.; Silva, M.L. Effects of Bothrops alternatus venom in zebrafish: A histopathological study. Inflammopharmacology 2017, 26, 273–284. [Google Scholar] [CrossRef]
- Dammski, A.P.; Müller, B.R.; Gaya, C.; Regonato, D. Zebrafish: Manual de Criação em Biotério; Universidade Federal do Paraná: Curitiba, Brazil, 2011. [Google Scholar]
- Holden, J.A.; Layfield, L.L.; Matthews, J.L. The Zebrafish: Atlas of Macroscopic and Microscopic Anatomy; Cambridge University Press: Cambridge, UK, 2012; 147p. [Google Scholar]
- Souza, G.C.; Duarte, J.L.; Fernandes, C.P.; Moyado, J.A.V.; Navarrete, A.; Carvalho, J.C.T. Obtainment and Study of the Toxicity of Perillyl Alcohol Nanoemulsion on Zebrafish (Danio rerio). J. Nanomed. Res. 2016, 4, 00093. [Google Scholar]
- Lins, J.A.P.N.; Kirschnik, P.G.; Queiroz, V.S.; Círio, S.M. Uso de peixes como biomarcadores para monitoramento ambiental aquático. Rev. Acad. Ciência Anim. 2010, 8, 469–484. [Google Scholar] [CrossRef]
- Funes, L.; Fernández-Arroyo, S.; Laporta, O.; Pons, A.; Roche, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Micol, V. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem. 2009, 117, 589–598. [Google Scholar] [CrossRef]
- Aguado, M.I.; Dudik, N.H.; Zamora, C.M.P.; Torres, C.A.; Nuñez, M.B. Antioxidant and antibacterial activities of hydroalcoholic extracts from Aloysia polystachya griseb moldenke and Lippia turbinata griseb (Verbenaceae). Int. J. Pharm. Pharm. Sci. 2016, 8, 393–395. [Google Scholar]
- Guo, Y.-P.; Lin, L.-G.; Wang, Y.-T. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. Chin. Med. 2015, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Abderrahim, F.; Estrella, S.; Susín, C.; Arribas, S.M.; González, M.C.; Condezo-Hoyos, L. The Antioxidant Activity and Thermal Stability of Lemon Verbena (Aloysia triphylla) Infusion. J. Med. Food 2011, 14, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Herranz-López, M.; Funes, L.; Borrás-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 2913, 20, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Sabzevari, A.; Mojarrab, M.; Nazemiyeh, H.; Esnaashari, S.; Nahar, L.; Razavi, S.M.; Sarker, S.D. Free-radical-scavenging principles from Phlomis caucasica. J. Nat. Med. 2008, 62, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.Y.F.; He, Z.-D.; Huang, Y.; Chen, Z.-Y. Antioxidative Activities of Phenylethanoid Glycosides from Ligustrum purpurascens. J. Agric. Food Chem. 2001, 49, 3113–3119. [Google Scholar] [CrossRef]
- Liu, M.J.; Li, J.X.; Guo, H.Z.; Lee, K.M.; Qin, L.; Chan, K.M. The effects of verbascoside on plasma lipid peroxidation level and erythrocyte membrane fluidity during immobilization in rabbits: A time course study. Life Sci. 2003, 73, 883–892. [Google Scholar] [CrossRef]
- Siciliano, T.; Bader, A.; Vassallo, A.; Braca, A.; Morelli, I.; Pizza, C.; De Tommasi, N. Secondary metabolites from Ballota undulata (Lamiaceae). Biochem. Syst. Ecol. 2005, 33, 341–351. [Google Scholar] [CrossRef]
- Ismailoglu, U.B.; Saracoglu, I.; Harput, U.S.; Sahin-Erdemli, I. Effects of phenylpropanoid and iridoid glycosides on free radical-induced impairment of endothelium-dependent relaxation in rat aortic rings. J. Ethnopharmacol. 2002, 79, 193–197. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Studies on the Antioxidant Activity of Lippia citriodora Infusion: Scavenging Effect on Superoxide Radical, Hydroxyl Radical and Hypochlorous Acid. Biol. Pharm. Bull. 2002, 25, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Seidel, V.; Verholle, M.; Malard, Y.; Tillequin, F.; Fruchart, J.C.; Duriez, P.; Bailleul, F.; Teissier, E. Phenylpropanoids from Ballota nigra L. inhibit in vitro LDL peroxidation. Phytother. Res. 2000, 14, 93–98. [Google Scholar] [CrossRef]
- Ohno, T.; Inoue, M.; Ogihara, Y.; Saracoglu, I. Antimetastatic activity of acteoside, a phenylethanoid glycoside. Biol. Pharm. Bull. 2002, 25, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Dal Toso, R.; Pressi, G.; Bramanti, P.; Meli, R.; Cuzzocrea, S. Protective effect of verbascoside in activated C6 glioma cells: Possible molecular mechanisms. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 381, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Obermeier, F.; Paper, D.H.; Balan, K.; Dunger, N.; Menzel, K.; Falk, W.; Schoelmerich, J.; Herfarth, H.; Rogler, G. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin. Exp. Immunol. 2007, 148, 373–381. [Google Scholar] [CrossRef]
- Schapoval, E.E.S.; Winter De Vargas, M.R.; Chaves, C.G.; Bridi, R.; Zuanazzi, J.A.; Henriques, A.T. Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis. J. Ethnopharmacol. 1998, 60, 53–59. [Google Scholar] [CrossRef]
- Wen, Y.; Huo, S.; Zhang, W.; Xing, H.; Qi, L.; Zhao, D.; Li, N.; Xu, J.; Yan, M.; Chen, X. Pharmacokinetics, Biodistribution, Excretion and Plasma Protein Binding Studies of Acteoside in Rats. Drug Res. 2015, 66, 148–153. [Google Scholar] [CrossRef]
- Serra, E.L.; Medalha, C.C.; Mattioli, R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz. J. Med. Biol. Res. 1999, 32, 1551–1553. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.; Wu, N.; Cachat, J.; Hart, P.; Gaikwad, S.; Wong, K.; Utterback, E.; Gilder, T.; Kyzar, E.; Newman, A.; et al. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A., Jr.; Zampieri, R.A.; Ramos, L.A.; Ferreira, E.; Silva, D.; Mattioli, R.; Morato, S. Preference of goldfish for dark places Preference of Goldfish (Carassius auratus) for Dark Places. Rev. Etol. 2005, 7, 63–66. [Google Scholar]
- Gouveia, A., Jr.; Maximino, C.; Brito, T. Comportamento de Peixes: Vantagens e Utilidades nas Neurociências; Faculdade de Ciências/UNESP: Bauru, São Paulo, Brazil, 2006; 80p. [Google Scholar]
- Benneh, C.K.; Biney, R.P.; Mante, P.K.; Tandoh, A.; Adongo, D.W.; Woode, E. Maerua angolensis stem bark extract reverses anxiety and related behaviours in zebrafish—Involvement of GABAergic and 5-HT systems. J. Ethnopharmacol. 2017, 207, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Da Silva, A.W.B.; Araujo, J.; Lima, M.G.; Miranda, V.; Puty, B.; Benzecry, R.; Picanco̧-Diniz, D.L.W.; Gouveia, A.; Oliveira, K.R.M.; et al. Fingerprinting of psychoactive drugs in zebrafish anxiety-like behaviors. PLoS ONE 2014, 9, 103943. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Silva, A.W.B.; Gouveia, A.; Herculano, A.M. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Marques, T.; Dias, F. A comparative analysis of the preference for dark environments in five teleosts. Int. J. Comp. Psychol. 2007, 20, 351–367. [Google Scholar]
- Cachat, J.M. Developing Zebrafish Models of Complex Phenotypes Relevant to Human Brain Disorders. Ph.D. Thesis, Tulane University School of Science and Engineering, New Orleans, LA, USA, 2013; pp. 1–259. [Google Scholar] [CrossRef]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magno, L.D.P.; Fontes, A.; Gonçalves, B.M.N.; Gouveia, A. Pharmacological study of the light–dark preference test in zebrafish (Danio rerio): Waterborne administration. Pharmacol. Biochem. Behav. 2015, 135, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.L.; Pagnussat, N.; Piato, Â.L.; Schaefer, I.C.; Bonan, C.D.; Lara, D.R. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol. Biochem. Behav. 2011, 99, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, C.; van der Linde, H.; Slieker, R.; Willemsen, R.; Wolterbeek, A.; Woutersen, R.; De Groot, D. Locomotor activity assay in zebrafish larvae: Influence of age, strain and ethanol. Neurotoxicol. Teratol. 2012, 34, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.D.; Soanes, K.H. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav. Brain Res. 2012, 233, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Vignet, C.; Bégout, M.-L.; Péan, S.; Lyphout, L.; Leguay, D.; Cousin, X. Systematic Screening of Behavioral Responses in Two Zebrafish Strains. Zebrafish 2013, 10, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramcharitar, J.; Ibrahim, R.M. Ethanol modifies zebrafish responses to abrupt changes in light intensity. J. Clin. Neurosci. 2013, 20, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.E.; Chadwick, L.; McGinnis, G.C. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.S.M. Investigação da Atividade Farmacológica Central dos Extratos Aquoso e Hidroalcoólico, da Fração Butanólica e do Verbascosídeo de Lippia alba (Miller) N. E. Brown (Falsa Melissa)—Verbenaceae. Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2006; 96p. [Google Scholar]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, T.I.S.; Melo, N.C.; Freitas Paiva, B.T.; Silva Aleluia, G.A.; Silva Neto, F.L.P.; Silva, H.R.; Keita, H.; Cruz, R.A.S.; Sánchez-Ortiz, B.L.; Pineda-Peña, E.A.; et al. Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: Pharmacological evaluation on zebrafish (Danio rerio). J. Ethnopharmacol. 2018, 224, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, L.J.G.; Ritter, F.; Kreutz, L.C.; Quevedo, R.M.; da Silva, L.B.; Bedin, A.C.; Finco, J.; Cericato, L. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 2007, 272, 774–778. [Google Scholar] [CrossRef]
- Abreu, M.S.; Giacomini, A.C.V.V.; Koakoski, G.; Piato, A.L.; Barcellos, L.J.G. Evaluating “anxiety” and social behavior in jundiá (Rhamdia quelen). Physiol. Behav. 2016, 160, 59–65. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.M.; Tien, D.H.; et al. Video-Aided analysis of Zebrafish locomotion and anxiety related behavioral responses. In Zebrafish Neurobehavioral Protocols; Human Press: Heidelberg, Germany, 2011; Volume 51, pp. 1–14. [Google Scholar] [CrossRef]
- Luca, R.M.; Gerlai, R. In search of optimal fear inducing stimuli: Differential behavioral responses to computer animated images in zebrafish. Behav. Brain Res. 2012, 226, 66–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathuru, A.S.; Kibat, C.; Cheong, W.F.; Shui, G.; Wenk, M.R.; Friedrich, R.W.; Jesuthasan, S. Chondroitin Fragments Are Odorants that Trigger Fear Behavior in Fish. Curr. Biol. 2012, 22, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, D.; Gerlai, R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav. Brain Res. 2009, 200, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, A.C.V.V.; Abreu, M.S.; Giacomini, L.V.; Siebel, A.M.; Zimerman, F.F.; Rambo, C.L.; Mocelin, R.; Bonan, C.D.; Piato, A.L.; Barcellos, L.J.G. Fluoxetine and diazepam acutely modulate stress induced-behavior. Behav. Brain Res. 2016, 296, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Picon, P.; Cosner, A.F.; Silveira, R.O. Relação entre estressores, estresse e ansiedade. Revista de Psiquiatria do Rio Grande do Sul 2003, 25, 65–74. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.H.; Janet, N.; Astrid, Z.; Edwin, E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [PubMed]
- Carvalho, T.S. A Intensificação do Comportamento Tipo Ansiedade Induzido por Cafeína em Danio rerio (Zebrafish) é Prevenida pelo Tratamento com α—Tocoferol e L-NAME. Master’s Thesis, Universidade Federal do Pará, Belém, Brazil, 2014; 105p. [Google Scholar]
- Agência Nacional de Vigilância Sanitária. Validação de Métodos Analíticos. Available online: http//www20.anvisa.gov.br/coifa/pdf/rdc166.pdf (accessed on 20 August 2018).
- Noakes, D.L.G.; Baylis, J.R. Behavior. Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 555–584. [Google Scholar]
- Tran, S.; Chatterjee, D.; Gerlai, R. Acute net stressor increases whole-body cortisol levels without altering whole-brain monoamines in zebrafish. Behav. Neurosci. 2014, 128, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.; Hylton, A. Behavioral, endocrine, and neuronal alterations in zebrafish (Danio rerio) following sub-chronic coadministration of fluoxetine and ketamine. Pharmacol. Biochem. Behav. 2015, 139, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.T.; Ichikawa, K.M. IPhone® applications as versatile video tracking tools to analyze behavior in zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2013, 106, 137–142. [Google Scholar] [CrossRef] [PubMed]















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa de Melo, N.; Sánchez-Ortiz, B.L.; dos Santos Sampaio, T.I.; Matias Pereira, A.C.; Pinheiro da Silva Neto, F.L.; Ribeiro da Silva, H.; Alves Soares Cruz, R.; Keita, H.; Soares Pereira, A.M.; Tavares Carvalho, J.C. Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 106. https://doi.org/10.3390/ph12030106
Costa de Melo N, Sánchez-Ortiz BL, dos Santos Sampaio TI, Matias Pereira AC, Pinheiro da Silva Neto FL, Ribeiro da Silva H, Alves Soares Cruz R, Keita H, Soares Pereira AM, Tavares Carvalho JC. Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio). Pharmaceuticals. 2019; 12(3):106. https://doi.org/10.3390/ph12030106
Chicago/Turabian StyleCosta de Melo, Nayara, Brenda Lorena Sánchez-Ortiz, Tafnis Ingret dos Santos Sampaio, Arlindo César Matias Pereira, Fernando Luiz Pinheiro da Silva Neto, Heitor Ribeiro da Silva, Rodrigo Alves Soares Cruz, Hady Keita, Ana Maria Soares Pereira, and José Carlos Tavares Carvalho. 2019. "Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio)" Pharmaceuticals 12, no. 3: 106. https://doi.org/10.3390/ph12030106
APA StyleCosta de Melo, N., Sánchez-Ortiz, B. L., dos Santos Sampaio, T. I., Matias Pereira, A. C., Pinheiro da Silva Neto, F. L., Ribeiro da Silva, H., Alves Soares Cruz, R., Keita, H., Soares Pereira, A. M., & Tavares Carvalho, J. C. (2019). Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio). Pharmaceuticals, 12(3), 106. https://doi.org/10.3390/ph12030106