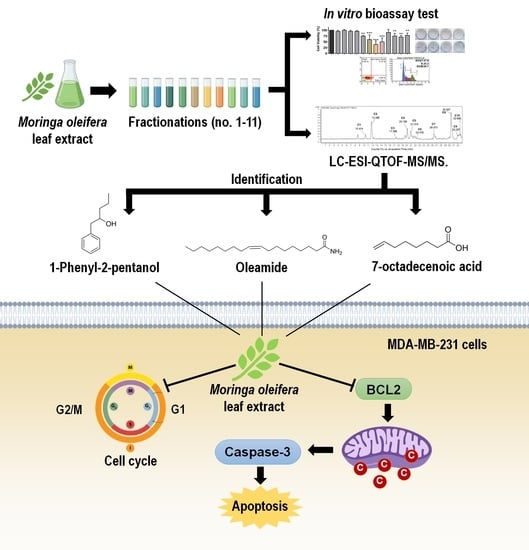
In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line
Abstract
:1. Introduction
2. Results
2.1. Screening for Cytotoxic Effects of Crude Hexane, EtOAc, and EtOH Extracts of MOL
2.2. Cytotoxic Effects of EtOAc Extract of MOL and Its Derived Fractions on MDA-MB-231 Cells
2.3. Effect of Crude EtOAc Extract and Fractions No. 6–8 on MDA-MB-231 Cell Apoptosis and Cell-Cycle Arrest
2.4. Sub-Fractionation of Fraction Bo. 7 and Identification of Compounds by LC-ESI-QTOF-MS/MS
2.5. The Role of Three-Identified Compounds, 7-Octenoic Acid, Oleamide, and 1-Phenyl-2-Pentanol on MDA-MB-231 Cells Apoptosis and Cell Cycle Progression
2.6. Bioactive Compounds Suppressed MDA-MB-231 Cell Migration and Induced Apoptosis in Different Cancer Cell Lines
2.7. Preliminary Prediction of Compound-Targets Interactions with Drug Target Commons
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Chemicals
4.2. Extraction and Fractionation of MOL Extract
4.3. At-Line-LC-ESI-QTOF-MS/MS Analysis
4.4. Identification of Active Compounds
4.5. Cell Viability Assay
4.6. Colony Formation Assay
4.7. Apoptosis and Cell Cycle Analysis
4.8. Cell Migration Assay
4.9. Hoechst Staining
4.10. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Breast Cancer Facts & Figures 2017–2018; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2018; American Cancer Society: Atlanta, GA, USA, 2018. [Google Scholar]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Andre, F.; Zielinski, C.C. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2012, 23 (Suppl. 6), vi46–vi51. [Google Scholar] [CrossRef]
- Odle, T.G. Adverse effects of breast cancer treatment. Radiol. Technol. 2014, 85, 297M–323M. [Google Scholar]
- Thurber, M.D.; Fahey, J.W. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the “Diffusion of innovations” theory. Ecol. Food Nutr. 2009, 48, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Dholvitayakhun, A.; Cushnie, T.P.; Trachoo, N. Antibacterial activity of three medicinal Thai plants against Campylobacter jejuni and other foodborne pathogens. Nat. Prod. Res. 2012, 26, 356–363. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I. Moringa oleifera Leaf Extracts as Multifunctional Ingredients for “Natural and Organic” Sunscreens and Photoprotective Preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [Green Version]
- Paikra, B.K.; Dhongade, H.K.J.; Gidwani, B. Phytochemistry and Pharmacology of Moringa oleifera Lam. J. Pharmacopunct. 2017, 20, 194–200. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N,alpha-L-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorganic Med. Chem. Lett. 2013, 23, 959–962. [Google Scholar] [CrossRef]
- Ghasi, S.; Nwobodo, E.; Ofili, J.O. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed wistar rats. J. Ethnopharmacol. 2000, 69, 21–25. [Google Scholar] [CrossRef]
- Ganguly, R.; Guha, D. Alteration of brain monoamines & EEG wave pattern in rat model of Alzheimer’s disease & protection by Moringa oleifera. Indian J. Med. Res. 2008, 128, 744–751. [Google Scholar]
- Cheenpracha, S.; Park, E.J.; Yoshida, W.Y.; Barit, C.; Wall, M.; Pezzuto, J.M.; Chang, L.C. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorganic Med. Chem. 2010, 18, 6598–6602. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Protective mechanisms of Moringa oleifera against CCl(4)-induced oxidative stress in precision-cut liver slices. Complementary Med. Res. 2010, 17, 189–194. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.H. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry 1995, 38, 957–963. [Google Scholar] [CrossRef]
- Mahajan, S.G.; Mehta, A.A. Inhibitory Action of Ethanolic Extract of Seeds of Moringa oleifera Lam. On Systemic and Local Anaphylaxis. J. Immunotoxicol. 2007, 4, 287–294. [Google Scholar] [CrossRef]
- Jaiswal, D.; Kumar Rai, P.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396. [Google Scholar] [CrossRef]
- Mbikay, M. Therapeutic Potential of Moringa oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front. Pharmacol. 2012, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Lurling, M.; Beekman, W. Anti-cyanobacterial activity of Moringa oleifera seeds. J. Appl. Phycol. 2010, 22, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Viera, G.H.; Mourao, J.A.; Angelo, A.M.; Costa, R.A.; Vieira, R.H. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev. Inst. Med. Trop. De Sao Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Sudha, P.; Asdaq, S.M.; Dhamingi, S.S.; Chandrakala, G.K. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in animals. Indian J. Physiol. Pharmacol. 2010, 54, 133–140. [Google Scholar]
- Gupta, A.; Gautam, M.K.; Singh, R.K.; Kumar, M.V.; Rao Ch, V.; Goel, R.K.; Anupurba, S. Immunomodulatory effect of Moringa oleifera Lam. extract on cyclophosphamide induced toxicity in mice. Indian J. Exp. Biol. 2010, 48, 1157–1160. [Google Scholar]
- Khor, K.Z.; Lim, V.; Moses, E.J.; Abdul Samad, N. The In Vitro and In Vivo Anticancer Properties of Moringa oleifera. Evid. Based Complementary Altern. Med. 2018, 2018, 1071243. [Google Scholar] [CrossRef] [Green Version]
- Madi, N.; Dany, M.; Abdoun, S.; Usta, J. Moringa oleifera’s Nutritious Aqueous Leaf Extract Has Anticancerous Effects by Compromising Mitochondrial Viability in an ROS-Dependent Manner. J. Am. Coll. Nutr. 2016, 35, 604–613. [Google Scholar] [CrossRef]
- Gismondi, A.; Canuti, L.; Impei, S.; Di Marco, G.; Kenzo, M.; Colizzi, V.; Canini, A. Antioxidant extracts of African medicinal plants induce cell cycle arrest and differentiation in B16F10 melanoma cells. Int. J. Oncol. 2013, 43, 956–964. [Google Scholar] [CrossRef]
- Nair, S.; Varalakshmi, K. Anticancer, cytotoxic potential of Moringa oleifera extracts on HeLa cell line. J. Nat. Pharm. 2011, 2, 138. [Google Scholar]
- Abd-Rabou, A.A.; Abdalla, A.M.; Ali, N.A.; Zoheir, K.M. Moringa oleifera Root Induces Cancer Apoptosis more Effectively than Leave Nanocomposites and Its Free Counterpart. Asian Pac. J. Cancer Prev. 2017, 18, 2141–2149. [Google Scholar] [CrossRef]
- Adebayo, I.A.; Arsad, H.; Samian, M.R. Antiproliferative effect on breast cancer (MCF7) of Moringa oleifera seed extracts. Afr. J. Tradit Complement. Altern. Med. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Tang, J.; Tanoli, Z.U.; Ravikumar, B.; Alam, Z.; Rebane, A.; Vähä-Koskela, M.; Peddinti, G.; van Adrichem, A.J.; Wakkinen, J.; Jaiswal, A.; et al. Drug Target Commons: A Community Effort to Build a Consensus Knowledge Base for Drug-Target Interactions. Cell Chem. Biol. 2018, 25, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Verdon, B.; Zheng, J.; Nicholson, R.A.; Ganelli, C.R.; Lees, G. Stereoselective modulatory actions of oleamide on GABA(A) receptors and voltage-gated Na(+) channels in vitro: A putative endogenous ligand for depressant drug sites in CNS. Br. J. Pharm. 2000, 129, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Sudhahar, V.; Shaw, S.; Imig, J.D. Mechanisms involved in oleamide-induced vasorelaxation in rat mesenteric resistance arteries. Eur. J. Pharm. 2009, 607, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.A.; Carson, M.J.; Neal, M.J.; Sutcliffe, J.G. Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide. Proc. Natl. Acad. Sci. USA 1997, 94, 14115–14119. [Google Scholar] [CrossRef] [Green Version]
- Boger, D.L.; Patterson, J.E.; Jin, Q. Structural requirements for 5-HT2A and 5-HT1A serotonin receptor potentiation by the biologically active lipid oleamide. Proc. Natl. Acad. Sci. USA 1998, 95, 4102–4107. [Google Scholar] [CrossRef] [Green Version]
- Kita, M.; Ano, Y.; Inoue, A.; Aoki, J. Identification of P2Y receptors involved in oleamide-suppressing inflammatory responses in murine microglia and human dendritic cells. Sci. Rep. 2019, 9, 3135. [Google Scholar] [CrossRef] [Green Version]
- Boger, D.L.; Fecik, R.A.; Patterson, J.E.; Miyauchi, H.; Patricelli, M.P.; Cravatt, B.F. Fatty acid amide hydrolase substrate specificity. Bioorganic Med. Chem. Lett. 2000, 10, 2613–2616. [Google Scholar] [CrossRef]
- Lambert, D.M.; Fowler, C.J. The endocannabinoid system: Drug targets, lead compounds, and potential therapeutic applications. J. Med. Chem. 2005, 48, 5059–5087. [Google Scholar] [CrossRef]
- Seierstad, M.; Breitenbucher, J.G. Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. J. Med. Chem. 2008, 51, 7327–7343. [Google Scholar] [CrossRef]
- Xu, M.Z.; Lee, W.S.; Kim, M.J.; Park, D.S.; Yu, H.; Tian, G.R.; Jeong, T.S.; Park, H.Y. Acyl-CoA: Cholesterol acyltransferase inhibitory activities of fatty acid amides isolated from Mylabris phalerate Pallas. Bioorganic Med. Chem. Lett. 2004, 14, 4277–4280. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.D.; Galbraith, M.D. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef] [Green Version]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, H.J.; Khan, N.A.; Mahmud, R.; Asmawi, M.Z.; Vikneswaran, A.; Murugaiyah, L. LC/MS, GC/MS screening and in vivo anti-inflammatory activity of Malaysian Moringa oleifera Lam leaf extracts and fractions against carrageenan -induced paw oedema in rats. J. Innov. Pharm. Biol. Sci. 2017, 4, 48–54. [Google Scholar]
- Karthivashan, G.; Tangestani Fard, M.; Arulselvan, P.; Abas, F.; Fakurazi, S. Identification of Bioactive Candidate Compounds Responsible for Oxidative Challenge from Hydro-Ethanolic Extract of Moringa oleifera Leaves. J. Food Sci. 2013, 78, C1368–C1375. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Wang, Z.; Li, P.; Zhao, C.; Liu, J. Comparative Analysis of Chemical Constituents of Moringa oleifera Leaves from China and India by Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-Of-Flight Mass Spectrometry. Molecules 2019, 24, 942. [Google Scholar] [CrossRef] [Green Version]
- Cravatt, B.F.; Prospero-Garcia, O.; Siuzdak, G.; Gilula, N.B.; Henriksen, S.J.; Boger, D.L.; Lerner, R.A. Chemical characterization of a family of brain lipids that induce sleep. Science 1995, 268, 1506–1509. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Sepe, N.; De Petrocellis, L.; Mechoulam, R.; Di Marzo, V. The sleep inducing factor oleamide is produced by mouse neuroblastoma cells. Biochem. Biophys. Res. Commun. 1997, 239, 473–479. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.A.; Hong, J.H.; Kim, J.S.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef]
- Zibara, K.; Awada, Z.; Dib, L.; El-Saghir, J.; Al-Ghadban, S.; Ibrik, A.; El-Zein, N.; El-Sabban, M. Anti-angiogenesis therapy and gap junction inhibition reduce MDA-MB-231 breast cancer cell invasion and metastasis in vitro and in vivo. Sci. Rep. 2015, 5, 12598. [Google Scholar] [CrossRef]
- Ito, A.; Morita, N.; Miura, D.; Koma, Y.; Kataoka, T.R.; Yamasaki, H.; Kitamura, Y.; Kita, Y.; Nojima, H. A derivative of oleamide potently inhibits the spontaneous metastasis of mouse melanoma BL6 cells. Carcinogenesis 2004, 25, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Sodvadiya, M.; Patel, H.; Mishra, A.; Nair, S. Emerging Insights into Anticancer Chemopreventive Activities of Nutraceutical Moringa oleifera: Molecular Mechanisms, Signal Transduction and In Vivo Efficacy. Curr. Pharmacol. Rep. 2020, 6, 38–51. [Google Scholar] [CrossRef]
- Van Swearingen, A.E.D.; Sambade, M.J.; Siegel, M.B.; Sud, S.; McNeill, R.S.; Bevill, S.M.; Chen, X.; Bash, R.E.; Mounsey, L.; Golitz, B.T.; et al. Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro-Oncol. 2017, 19, 1481–1493. [Google Scholar] [CrossRef]
- McLaughlin, R.P.; He, J.; van der Noord, V.E.; Redel, J.; Foekens, J.A.; Martens, J.W.M.; Smid, M.; Zhang, Y.; van de Water, B. A kinase inhibitor screen identifies a dual cdc7/CDK9 inhibitor to sensitise triple-negative breast cancer to EGFR-targeted therapy. Breast Cancer Res. 2019, 21, 77. [Google Scholar] [CrossRef]
- Tang, J.; Gautam, P.; Gupta, A.; He, L.; Timonen, S.; Akimov, Y.; Wang, W.; Szwajda, A.; Jaiswal, A.; Turei, D.; et al. Network pharmacology modeling identifies synergistic Aurora B and ZAK interaction in triple-negative breast cancer. NPJ Syst. Biol. Appl. 2019, 5, 20. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Crystal, A.S.; Shaw, A.T.; Sequist, L.V.; Friboulet, L.; Niederst, M.J.; Lockerman, E.L.; Frias, R.L.; Gainor, J.F.; Amzallag, A.; Greninger, P.; et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014, 346, 1480–1486. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.S.; Asif, M.; Basheer, M.K.A.; Kang, C.W.; Al-Suede, F.S.; Ein, O.C.; Tang, J.; Majid, A.S.A.; Majid, A. Treatment of novel IL17A inhibitor in glioblastoma implementing 3rd generation co-culture cell line and patient-derived tumor model. Eur. J. Pharm. 2017, 803, 24–38. [Google Scholar] [CrossRef]
- He, L.; Tang, J.; Andersson, E.I.; Timonen, S.; Koschmieder, S.; Wennerberg, K.; Mustjoki, S.; Aittokallio, T. Patient-Customized Drug Combination Prediction and Testing for T-cell Prolymphocytic Leukemia Patients. Cancer Res. 2018, 78, 2407–2418. [Google Scholar] [CrossRef] [Green Version]









| No. | RT (min) | m/z [M + H]+ | MS/MS | Tentative Identification | Formula | Error (ppm) |
|---|---|---|---|---|---|---|
| C1 | 10.414 | 197.1166 | 179.1015, 161.0911, 135.1127, 107.0822 | 2 -(benzyloxy) butane-1,4-diol | C11H16O3 | 3.15 |
| C2 | 12.286 | 143.1057 | 128.0550, 101.0912, 83.0814, 62.9783, 59.0458, 55.0513 | 7-octenoic acid | C8H14O2 | 6.68 |
| C3 | 17.586 | 165.1272 | 147.1157, 95.0482 | 1-Phenyl-2-pentanol | C11H16O | 1.16 |
| C4 | 20.198 | 291.1955 | 273.1828 | 8-oxo-9,11-octadecadiynoic acid | C18H26O3 | −0.79 |
| C5 | 21.015 | 291.1958 | 273.1806, 171.1019 | 4-oxo-octadeca-9Z,11E,13E,15Z-tetraenoic acid, Chrysobalanic acid | C18H26O3 | −1.13 |
| C6 | 22.476 | 325.2013 | 291.1925, 233.1518, 137.0949 | 12-oxo-14,18-dihydroxy-9Z,13E,15Z-octadecatrienoic acid | C18H28O5 | −1.08 |
| C7 | 26.473 | 277.2148 | 135.1125, 93.0669, 79.0517 | 3E,9Z,12Z,15Z-Octadecatetraenoic acid | C18H28O2 | 5.09 |
| C8 | 30.557 | 372.3457 | 354.3303, 337.3052, 319.2933, 97.0993, 83.0840 | 13,14-dihydroxydocosanamide | C22H45NO3 | 2.75 |
| C9 | 32.057 | 354.3379 | 337.3075, 319.2965, 301.2865 | N-(11Z-eicosaenoyl)-ethanolamine | C22H43NO2 | −3.51 |
| C10 | 32.606 | 282.2784 | * | 9Z-octadecenamide | C18H35NO | 2.63 |
| Target Preferred Name | Gene Names | Target Class | References |
|---|---|---|---|
| 1. GABA-A receptor β3 subunit | GABRB3 | Ion channel | [35,36] |
| 2. Cannabinoid receptor 1 (CB1) | CNR1 | GPCR | [36] |
| 3. 5-HT2A receptor | HTR2A | GPCR | [37,38] |
| 4. P2Y receptors | P2RY | GPCR | [39] |
| 5. Fatty acid amide hydrolase | FAAH | Enzyme | [40,41,42] |
| 6. Acyl coenzyme A: cholesterol acyltransferase 1 | Soat1 | Enzyme | [43] |
| 7. Cytochrome p450 2c19 | Cyp2c19 | Enzyme | Pubchem bioassay |
| 8. Cytochrome p450 2c19 | Cyp2c19 | Enzyme | Pubchem bioassay |
| 9. Cytochrome P450 2C9 | Cyp2c9 | Enzyme | Pubchem bioassay |
| 10. Cytochrome P450 2D6 | Cyp2d6 | Enzyme | Pubchem bioassay |
| 11. Cytochrome P450 3A4 | Cyp3a4 | Enzyme | Pubchem bioassay |
| 12. Cytochrome p450 1a2 | Cyp1a2 | Enzyme | Pubchem bioassay |
| 13. Ubiquitin carboxyl-terminal hydrolase 1 | Usp1 | Kinase | Pubchem bioassay |
| 14. NF-kappa-B, p105 subunit | Nfkb1 | Nuclear receptor | Pubchem bioassay |
| 15. Proto-oncogene c-jun | Jun | Nuclear receptor | Pubchem bioassay |
| 16. Cellular tumor antigen p53 | Tp53 | Nuclear receptor | Pubchem bioassay |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 464. https://doi.org/10.3390/ph13120464
Wisitpongpun P, Suphrom N, Potup P, Nuengchamnong N, Calder PC, Usuwanthim K. In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line. Pharmaceuticals. 2020; 13(12):464. https://doi.org/10.3390/ph13120464
Chicago/Turabian StyleWisitpongpun, Prapakorn, Nungruthai Suphrom, Pachuen Potup, Nitra Nuengchamnong, Philip C. Calder, and Kanchana Usuwanthim. 2020. "In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line" Pharmaceuticals 13, no. 12: 464. https://doi.org/10.3390/ph13120464
APA StyleWisitpongpun, P., Suphrom, N., Potup, P., Nuengchamnong, N., Calder, P. C., & Usuwanthim, K. (2020). In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line. Pharmaceuticals, 13(12), 464. https://doi.org/10.3390/ph13120464