Epigallocatechin-3-Gallate-Loaded Gold Nanoparticles: Preparation and Evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of EGCG-GNPs
2.2. Effect of EGCG/HAuCl4 Molar Ratio on GNPs Properties
2.3. Effect of EGCG/HAuCl4 Molar Ratio on GNPS Size and Polydispersity Index
2.4. Effect of EGCG/HAuCl4 Molar Ratio on Drug Loading Properties
2.5. Fourier-Transform (FT-IR) Spectroscopy Studies
2.6. Differential Scanning Calorimetry (DSC) Studies
2.7. In Vitro Drug Release Studies
2.8. Storage Stability Studies
2.9. Hemocompatibility Studies
2.9.1. Hemolysis Studies
2.9.2. Effect of EGCG and EGCG-GNPs on Prothrombin Time and Partial Thromboplastin Time
2.9.3. Effect of EGCG and EGCG-GNPs on Complement Protein (C3)
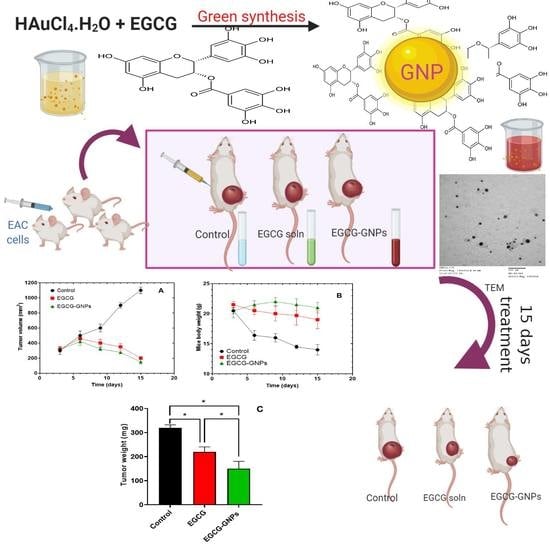
2.10. In Vivo Studies
3. Materials and Methods
3.1. Materials
3.2. Fabrication of EGCG-Loaded Gold Nanoparticles (EGCG-GNPs)
3.3. Determination of Drug Encapsulation Efficiency
3.4. Particle Size and Zeta Potential Measurements
3.5. Transmission Electron Microscope (TEM) Measurements
3.6. Fourier-Transform Infrared (FT-IR) Spectroscopy Studies
3.7. Differential Scanning Calorimetry (DSC) Studies
3.8. In Vitro Drug Release Studies
3.9. Storage Stability Study
3.10. Hemocompatibility Studies
3.10.1. Hemolysis Test
3.10.2. Partial Thromboplastin Time (PTT) and Prothrombin Time (PT) Assay
3.10.3. Complement Activation by the Nephelometric Method
3.11. In Vivo Studies
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Cancer Fact Sheets; World Health Organization: Geneve, Switzerland, 2018. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.; Kavan, P.; Miller, W.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.; et al. Systemic cancer therapy: Achievements and challenges that lie ahead. Front. Pharm. 2013, 4, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Hsieh, D.S.; Huang, K.J.; Chan, Y.L.; Hong, P.D.; Yeh, M.K.; Wu, C.J. Improving anticancer efficacy of (–)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells. Drug Des. Devel. Ther. 2014, 8, 459–474. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Adachi, S.; Masuda, M.; Kozawa, O.; Moriwaki, H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol. Nutr. Food Res. 2011, 55, 832–843. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Anti-inflamm. Anti-allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Tauber, A.L.; Schweiker, S.S.; Levonis, S.M. From tea to treatment; epigallocatechin gallate and its potential involvement in minimizing the metabolic changes in cancer. Nutr. Res. 2020, 74, 23–36. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Han, Y.; Chen, C.; Sun, H.; He, D.; Guo, J.; Jiang, B.; Zhou, L.; Zeng, C. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-κB signaling in human umbilical vein endothelial cells. Life Sci. 2013, 92, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S. Chronic inflammatory diseases and green tea polyphenols. Nutrients 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.J.; Qiao, K.S.; Sun, P.; Chen, P.; Li, Q. Study of EGCG induced apoptosis in lung cancer cells by inhibiting PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4557–4563. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Adachi, S.; Sakai, H.; Nakagawa, T.; Yasuda, Y.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (–)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor–vascular endothelial growth factor receptor axis. Cancer Sci. 2009, 100, 1957–1962. [Google Scholar] [CrossRef]
- Ahmad, N.; Gupta, S.; Mukhtar, H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor κB in cancer cells versus normal cells. Arch. Biochem. Biophys. 2000, 376, 338–346. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Lambert, J.D.; Lee, M.-J.; Lu, H.; Meng, X.; Hong, J.J.J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zhang, L.; Zuo, Z. Intestinal efflux transport kinetics of green tea catechins in Caco-2 monolayer model. J. Pharm. Pharmacol. 2007, 59, 395–400. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, S.; Das, D.K.; Chakraborty, P.; Choudhury, S.; Gupta, P.; Adhikary, A.; Dey, S.; Chattopadhyay, S. Gold-conjugated green tea nanoparticles for enhanced anti-tumor activities and hepatoprotection—Synthesis, characterization and in vitro evaluation. J. Nutr. Biochem. 2015, 26, 1283–1297. [Google Scholar] [CrossRef]
- Sun, M.; Xie, Q.; Cai, X.; Liu, Z.; Wang, Y.; Dong, X.; Xu, Y. Preparation and characterization of epigallocatechin gallate, ascorbic acid, gelatin, chitosan nanoparticles and their beneficial effect on wound healing of diabetic mice. Int. J. Biol. Macromol. 2020, 148, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ruan, C.; Sun, Y.; Gao, X.; Liang, J. Controlled release and antioxidant activity of chitosan and β-lactoglobulin complex nanoparticles loaded with epigallocatechin gallate. Colloids Surf. B Biointerfaces 2020, 188, 110802. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm. Dev. Technol. 2019, 24, 1243–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Zhu, L.; Yu, J.; Wang, Y.; Peng, B. Anti-osteoclastogenic effect of epigallocatechin gallate-functionalized gold nanoparticles in vitro and in vivo. Int. J. Nanomed. 2019, 14, 5017–5032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granja, A.; Neves, A.R.; Sousa, C.T.; Pinheiro, M.; Reis, S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon 2019, 5, e02020. [Google Scholar] [CrossRef] [Green Version]
- Aborig, M.; Malik, P.R.V.; Nambiar, S.; Chelle, P.; Darko, J.; Mutsaers, A.; Edginton, A.N.; Fleck, A.; Osei, E.; Wettig, S. Biodistribution and physiologically-based pharmacokinetic modeling of gold nanoparticles in mice with interspecies extrapolation. Pharmaceutics 2019, 11, 179. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, W.; Tu, G.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Enhanced chemotherapeutic efficacy of PLGA-encapsulated epigallocatechin gallate (EGCG) against human lung cancer. Int. J. Nanomed. 2020, 15, 4417–4429. [Google Scholar]
- Hsieh, D.S.; Wang, H.; Tan, S.W.; Huang, Y.H.; Tsai, C.Y.; Yeh, M.K.; Wu, C.J. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials 2011, 32, 7633–7640. [Google Scholar] [CrossRef]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [Green Version]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Fluorouracil-loaded gold nanoparticles for the treatment of skin cancer: Development, in vitro characterization, and in vivo evaluation in a mouse skin cancer xenograft model. Mol. Pharm. 2018, 15, 2194–2205. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (aunps) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Mioc, A.; Mioc, M.; Ghiulai, R.; Voicu, M.; Racoviceanu, R.; Trandafirescu, C.; Dehelean, C.; Coricovac, D.; Soica, C. Gold nanoparticles as targeted delivery systems and theranostic agents in cancer therapy. Curr. Med. Chem. 2019, 26, 6493–6513. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Fathalla, D.; Youssef, E.M.; Soliman, G.M. Liposomal and ethosomal gels for the topical delivery of anthralin: Preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics 2020, 12, 446. [Google Scholar] [CrossRef]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Gold nanoparticles capped with benzalkonium chloride and poly (ethylene imine) for enhanced loading and skin permeability of 5-fluorouracil. Drug Dev. Ind. Pharm. 2017, 43, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin gallate-gold nanoparticles exhibit superior antitumor activity compared to conventional gold nanoparticles: Potential synergistic interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 2019, 311–312, 170–189. [Google Scholar] [CrossRef]
- Rane, T.D.; Armani, A.M. Two-photon microscopy analysis of gold nanoparticle uptake in 3D cell spheroids. PLoS ONE 2016, 11, e0167548. [Google Scholar] [CrossRef]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan gum stabilized gold nanoparticles: Characterization, biocompatibility, stability and cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar] [CrossRef]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, G.M.; Attia, M.A.; Mohamed, R.A. Poly(ethylene glycol)-block-poly(epsilon-caprolactone) nanomicelles for the solubilization and enhancement of antifungal activity of sertaconazole. Curr. Drug Deliv. 2014, 11, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masse, F.; Desjardins, P.; Ouellette, M.; Couture, C.; Omar, M.M.; Pernet, V.; Guérin, S.; Boisselier, E. Synthesis of ultrastable gold nanoparticles as a new drug delivery system. Molecules 2019, 24, 2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C. Group wavenumbers and an introduction to the spectroscopy of benzene rings. Spectroscopy 2016, 31, 34–37. [Google Scholar]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Meng, D.L.; Shang, L.; Feng, X.H.; Huang, X.F.; Che, X. Xanthoceraside hollow gold nanoparticles, green pharmaceutics preparation for poorly water-soluble natural anti-AD medicine. Int. J. Pharm. 2016, 506, 184–190. [Google Scholar] [CrossRef]
- Sun, Y.W.; Wang, L.H.; Meng, D.L.; Che, X. A green and facile preparation approach, licochalcone A capped on hollow gold nanoparticles, for improving the solubility and dissolution of anticancer natural product. Oncotarget 2017, 8, 105673–105681. [Google Scholar] [CrossRef] [PubMed]
- De la Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The hemocompatibility of nanoparticles: A review of cell-nanoparticle interactions and hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-W.; Powers, K.; Baney, R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr. Polym. 2004, 58, 371–377. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Love, S.A.; Thompson, J.W.; Haynes, C.L. Development of screening assays for nanoparticle toxicity assessment in human blood: Preliminary studies with charged Au nanoparticles. Nanomedicine 2012, 7, 1355–1364. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef]
- Oslakovic, C.; Cedervall, T.; Linse, S.; Dahlbäck, B. Polystyrene nanoparticles affecting blood coagulation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 981–986. [Google Scholar] [CrossRef]
- Mayer, A.; Vadon, M.; Rinner, B.; Novak, A.; Wintersteiger, R.; Fröhlich, E. The role of nanoparticle size in hemocompatibility. Toxicology 2009, 258, 139–147. [Google Scholar] [CrossRef]
- Pagana, K.D.; Pagana, T.J. Mosby’s Diagnostic and Laboratory Test Reference-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Janssen, B.J.; Huizinga, E.G.; Raaijmakers, H.C.; Roos, A.; Daha, M.R.; Nilsson-Ekdahl, K.; Nilsson, B.; Gros, P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 2005, 437, 505–511. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.-S.; Lu, H.-C.; Chen, C.-C.; Wu, C.-J.; Yeh, M.-K. The preparation and characterization of gold-conjugated polyphenol nanoparticles as a novel delivery system. Int. J. Nanomed. 2012, 7, 1623–1633. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.; Neun, B. Analysis of hemolytic properties of nanoparticles. In NCL Method ITA-1 Version 1.1; Nanotechnology Characterization Laboratory: Frederick, MD, USA, 2009. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices; Part 4: Selection of Tests for Interactions with Blood; International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- McNeil, S.E. Characterization of Nanoparticles Intended for Drug Delivery; Springer: New York, NY, USA, 2011; Volume 697. [Google Scholar]
- Thasneem, Y.M.; Sajeesh, S.; Sharma, C.P. Effect of thiol functionalization on the hemo-compatibility of PLGA nanoparticles. J. Biomed. Mater. Res. Part A 2011, 99, 607–617. [Google Scholar] [CrossRef] [PubMed]









| Formula | EGCG/HAuCl4 a | EE (%) b | LC (%) c | Z- Average Size (nm) d | PDI e |
|---|---|---|---|---|---|
| F1 | 0.2:1 | 72 ± 7.7 | 15.2 ± 1.4 | 26.3 ± 2.4 | 0.55 ± 0.01 |
| F2 | 0.4:1 | 92.6 ± 0.6 | 31.6 ± 0.1 | 33.8 ± 2.2 | 0.33 ± 0.04 |
| F3 | 0.8:1 | 32.5 ± 5.4 | 24.4 ± 3.1 | 43.8 ± 1.7 | 0.46 ± 0.02 |
| F4 | 1.6:1 | 18.1 ± 0.4 | 26.6 ± 0.4 | 332.9 ± 10.5 | 0.57 ± 0.03 |
| F5 | 3.1:1 | 26.1 ± 2.2 | 51 ± 2.1 | 56.9 ± 3.6 | 0.42 ± 0.05 |
| F6 | 4.7:1 | 26.9 ± 13.9 | 58.8 ± 13.9 | 610.1 ± 20.7 | 0.78 ± 0.10 |
| Time | Encapsulation Efficiency (%) | Z- Average Size (nm) | PDI | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|---|---|
| 4 °C | RT | 4 °C | RT | 4 °C | RT | 4 °C | RT | |
| Zero time | 92.6 ± 0.6 | 92.6 ± 0.6 | 33.8 ± 2.2 | 33.8 ± 2.2 | 0.3 ± 0.04 | 0.3 ± 0.04 | −24.9 ± 0.3 | −24.9 ± 0.3 |
| 1 month | 90.8 ± 0.4 | 89.7 ± 0.2 * | 37.9 ± 1.4 * | 32.3 ± 0.9 | 0.4 ± 0.0 | 0.4 ± 0.1 | −27.1 ± 1.3 * | −23.3 ± 1.4 * |
| 2 months | 90.5 ± 0.6 | 86.7 ± 0.2 * | 30.3 ± 0.8 * | 30.3 ± 0.3 | 0.3 ± 0.0 | 0.3 ± 0.0 | −25.4 ± 0.8 | −27.1 ± 1.0 * |
| 3 months | 91.3 ± 1.6 | 89.7 ± 0.8 * | 44.2 ± 0.3 * | 30.7 ± 1.9 | 0.4 ± 0.0 | 0.3 ± 0.1 | −28.0 ± 0.6 * | −28.3 ± 0.3 * |
| 4 months | 86.7 ± 1.2 * | 81.1 ± 1.6 * | 33.7 ± 1.0 | 31.8 ± 0.5 | 0.3 ± 0.0 | 0.3 ± 0.0 | −25.1 ± 0.4 | −26.1 ± 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safwat, M.A.; Kandil, B.A.; Elblbesy, M.A.; Soliman, G.M.; Eleraky, N.E. Epigallocatechin-3-Gallate-Loaded Gold Nanoparticles: Preparation and Evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing Mice. Pharmaceuticals 2020, 13, 254. https://doi.org/10.3390/ph13090254
Safwat MA, Kandil BA, Elblbesy MA, Soliman GM, Eleraky NE. Epigallocatechin-3-Gallate-Loaded Gold Nanoparticles: Preparation and Evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing Mice. Pharmaceuticals. 2020; 13(9):254. https://doi.org/10.3390/ph13090254
Chicago/Turabian StyleSafwat, Mohamed A., Bothaina A. Kandil, Mohamed A. Elblbesy, Ghareb M. Soliman, and Nermin E. Eleraky. 2020. "Epigallocatechin-3-Gallate-Loaded Gold Nanoparticles: Preparation and Evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing Mice" Pharmaceuticals 13, no. 9: 254. https://doi.org/10.3390/ph13090254
APA StyleSafwat, M. A., Kandil, B. A., Elblbesy, M. A., Soliman, G. M., & Eleraky, N. E. (2020). Epigallocatechin-3-Gallate-Loaded Gold Nanoparticles: Preparation and Evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing Mice. Pharmaceuticals, 13(9), 254. https://doi.org/10.3390/ph13090254