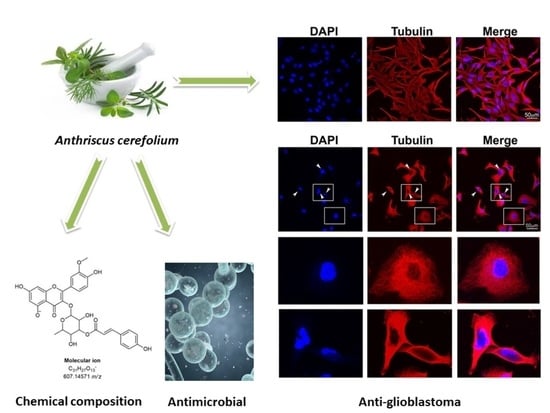
Extract of Herba Anthrisci cerefolii: Chemical Profiling and Insights into Its Anti-Glioblastoma and Antimicrobial Mechanism of Actions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Phenolic Acids and Flavonoids
2.2. Anti-Glioblastoma Activity
2.3. Antimicrobial Activities of Herba Anthrisci cerefolii
3. Materials and Methods
3.1. Collection and Extraction of Plant Material
3.2. Chemicals and Reagents
3.3. UHPLC-LTQ OrbiTrap MS4 Analysis of Phenolic Acid and Flavonoid Derivatives
3.4. Investigation of the Antiproliferative Effect of A. cerefolium Extract
3.5. Immunocytochemistry
3.6. Antimicrobial Susceptibility Tests
3.7. Activity against Formation and Inhibition of Microbial Biofilms
3.8. Insights into Modes of Antibacterial and Antifungal Actions of Extract
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luwor, R.B.; Stylli, S.S.; Kaye, A.H. The role of Stat3 in glioblastoma multiforme. J. Clin. Neurosci. 2013, 20, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neagu, M.R.; Reardon, D.A. Rindopepimut vaccine and bevacizumab combination therapy: Improving survival rates in relapsed glioblastoma patients? Immunotherapy 2015, 7, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Rasul, F.T.; Watts, C. Novel Surgical Approaches to High-Grade Gliomas. Curr. Treat. Options Neurol. 2015, 17, 38. [Google Scholar] [CrossRef]
- Zanders, E.D.; Svensson, F.; Bailey, D.S. Therapy for glioblastoma: Is it working? Drug Discov. Today 2019, 24, 1193–1201. [Google Scholar] [CrossRef]
- Eyvazi, S.; Vostakolaei, M.A.; Dilmaghani, A.; Borumandi, O.; Hejazi, M.S.; Kahroba, H.; Tarhriz, V. The oncogenic roles of bacterial infections in development of cancer. Microb. Pathog. 2020, 141, 104019. [Google Scholar] [CrossRef]
- Chang, A.H.; Parsonnet, J. Role of bacteria in oncogenesis. Clin. Microbiol. Rev. 2010, 23, 837–857. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-De-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar] [CrossRef]
- Bai, C.; Li, D.; Zhang, Q.; Zheng, S.; Li, Z.; Wang, H.; Li, L.; Zhang, W. Prognostic analysis of cancer patients with Staphylococcus aureus infection: Five-year experience at a comprehensive cancer center. Int. J. Clin. Exp. Med. 2018, 11, 8640–8645. [Google Scholar]
- Vyas, A.; Shukla, S.S.; Pandey, R.; Jain, V.; Joshi, V.; Gidwani, B. Chervil: A multifunctional miraculous nutritional herb. Asian J. Plant Sci. 2012, 11, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, A.A.; Srinivasappa, K.N. Chervil. In Handbook of Herbs and Spices, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 2, pp. 268–274. ISBN 9780857095688. [Google Scholar]
- Milovanovic, M.; Picuric-Jovanovic, K.; Vucelic-Radovic, B.; Vrbaski, Z. Antioxidant effects of flavonoids of Anthriscus sylvestris in lard. JAOCS J. Am. Oil Chem. Soc. 1996, 73, 773–776. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Giorgetti, M.; Cervellati, R.; Innocenti, G. Deoxypodophyllotoxin content and antioxidant activity of aerial parts of Anthriscus sylvestris Hoffm. Z. Für Nat. C 2006, 61, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R. Composition of the essential oils from Peucedanum cervaria and P. alsaticum growing wild in the Urban Area of Vienna (Austria). Nat. Prod. Commun. 2012, 7, 1515–1518. [Google Scholar] [CrossRef] [Green Version]
- Hendrawati, O.; Woerdenbag, H.J.; Michiels, P.J.A.; Aantjes, H.G.; Van Dam, A.; Kayser, O. Identification of lignans and related compounds in Anthriscus sylvestris by LC-ESI-MS/MS and LC-SPE-NMR. Phytochemistry 2011, 72, 2172–2179. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Jekő, J.; Cziáky, Z.; et al. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study. Ind. Crops Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Sekhara, I.; Benaissa, O.; Amrani, A.; Giangiacomo, B.; Benabderrahmane, W.; Chaouch, M.A.; Zama, D.; Benayache, S.; Benayache, F. Antioxidant activity and chemical constituents of Anthriscus vulgaris Bernh. (Apiaceae) from Algeria. Acta Sci. Nat. 2020, 7, 59–70. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Sytar, O.; Dursunoglu, B.; Ozbek, H.; Duman, H.; Guvenalp, Z.; Kılıc, C.S. Antioxidant and anticholinesterase potential of Ferulago cassia with farther bio-guided isolation of active coumarin constituents. S. Afr. J. Bot. 2019, 121, 536–542. [Google Scholar] [CrossRef]
- de la Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Bouratoua, A.; Khalfallah, A.; Bensouici, C.; Kabouche, Z.; Alabdul Magid, A.; Harakat, D.; Voutquenne-Nazabadioko, L.; Kabouche, A. Chemical composition and antioxidant activity of aerial parts of Ferula longipes Coss. ex Bonnier and Maury. Nat. Prod. Res. 2018, 32, 1873–1880. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, P.; Qu, H.; Cheng, Y. Characterization of phenolic compounds in Erigeron breviscapus by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2971–2984. [Google Scholar] [CrossRef] [PubMed]
- Žemlička, L.; Fodran, P.; Lukeš, V.; Vagánek, A.; Slováková, M.; Staško, A.; Dubaj, T.; Liptaj, T.; Karabín, M.; Birošová, L.; et al. Physicochemical and biological properties of luteolin-7-O-β-d- glucoside (cynaroside) isolated from Anthriscus sylvestris (L.) Hoffm. Mon. Fur Chem. 2014, 145, 1307–1318. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Phytochemical Profile, Chemotaxonomic Studies, and In Vitro Antioxidant Activities of Two Endemisms from Madeira Archipelago: Melanoselinum decipiens and Monizia edulis (Apiaceae). Chem. Biodivers. 2016, 13, 1290–1306. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Kikuchi, M. Studies on the constituents of Anthriscus sylvestris Hoffm. II. On the components of the flowers and leaves. Yakugaku Zasshi 1979, 99, 602–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Yue, D.; Liu, D.; Liu, X.; Song, S. Chemical constituents from Bupleurum chinese and their chemotaxonomic significance. Biochem. Syst. Ecol. 2019, 86, 103929. [Google Scholar] [CrossRef]
- Nazemiyeh, H.; Delazar, A.; Movahedin, N.; Jodari, M.; Imani, Y.; Ghahramani, M.-A.; Nahar, L.; Sarker, S.D. Free radical scavengers from the aerial parts of Grammosciadium platycarpum Boiss. & Hausskn. (Apiaceae) and GC-MS analysis of the essential oils from its fruits. Rev. Bras. Farmacogn. 2009, 19, 914–918. [Google Scholar] [CrossRef] [Green Version]
- Abou El-Kassem, L.; Hawas, U.; Awad, H.; Taie, H. Flavonoids from the aerial parts of Eryngium campestre L. with antioxidant and anti-alzheimer activities. Planta Med. 2013, 79, PJ2. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Ownby, S.; Wang, P.; Yuan, W.; Zhang, W.; Scott Beasley, R. Phenolic compounds and rare polyhydroxylated triterpenoid saponins from Eryngium yuccifolium. Phytochemistry 2008, 69, 2070–2080. [Google Scholar] [CrossRef]
- Soliman, F.M.; Shehata, A.H.; Khaleel, A.E.; Ezzat, S.M. An acylated kaempferol glycoside from flowers of Foeniculum vulgare and F. dulce. Molecules 2002, 7, 245–251. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS analysis and biological properties of Origanum vulgare subsp. viridulum obtained by different extraction methods. Ind. Crops Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- Shrestha, A.; Hakeem Said, I.; Grimbs, A.; Thielen, N.; Lansing, L.; Schepker, H.; Kuhnert, N. Determination of hydroxycinnamic acids present in Rhododendron species. Phytochemistry 2017, 144, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, R.; Shopna, R. Isolation of Quercetin and Isorhamnetin Derivatives and Evaluation of Anti-microbial and Anti-inflammatory Activities of Persicaria glabra. Nat. Prod. Sci. 2015, 21, 170–175. [Google Scholar]
- Jovanova, B.; Kulevanova, S.; Kadifkova Panovska, T. Determination of the total phenolic content, antioxidant activity and cytotoxicity of selected aromatic herbs. Agric. Conspec. Sci. 2019, 84, 51–58. [Google Scholar]
- Lee, S.A.; Moon, S.-M.; Han, S.H.; Hwang, E.J.; Hong, J.H.; Park, B.-R.; Choi, M.S.; Ahn, H.; Kim, J.-S.; Kim, H.-J.; et al. In Vivo and in Vitro Anti-Inflammatory Effects of Aqueous Extract of Anthriscus sylvestris Leaves. J. Med. Food 2018, 21, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, P.; Rao, H.; Gao, Y. Chemical composition, cytotoxic, antimicrobial and antioxidant activities of essential oil from Anthriscus caucalis M. Bieb grown in China. Rec. Nat. Prod. 2018, 12, 290–294. [Google Scholar] [CrossRef]
- Pascale, M.; Aversa, C.; Barbazza, R.; Marongiu, B.; Siracusano, S.; Stoffel, F.; Sulfaro, S.; Roggero, E.; Bonin, S.; Stanta, G. The proliferation marker Ki67, but not neuroendocrine expression, is an independent factor in the prediction of prognosis of primary prostate cancer patients. Radiol. Oncol. 2016, 50, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armocida, D.; Pesce, A.; Di Giammarco, F.; Frati, A.; Salvati, M.; Santoro, A. Histological, molecular, clinical and outcomes characteristics of Multiple Lesion Glioblastoma. A retrospective monocentric study and review of literature. Neurocirugia 2020. [Google Scholar] [CrossRef] [PubMed]
- Vidak, M.; Rozman, D.; Komel, R. Effects of flavonoids from food and dietary supplements on glial and glioblastoma multiforme cells. Molecules 2015, 20, 19406–19432. [Google Scholar] [CrossRef]
- Pavlović, M.; Petrović, S.; Milenković, M.; Couladis, M.; Tzakou, O.; Niketić, M. Chemical composition and antimicrobial activity of Anthriscus nemorosa root essential oil. Nat. Prod. Commun. 2011, 6, 271–273. [Google Scholar] [CrossRef] [Green Version]
- Stojković, D.; Drakulić, D.; Gašić, U.; Zengin, G.; Stevanović, M.; Rajčević, N.; Soković, M. Ononis spinosa L., an edible and medicinal plant: UHPLC-LTQ-Orbitrap/MS chemical profiling and biological activities of the herbal extract. Food Funct. 2020, 11, 7138–7151. [Google Scholar] [CrossRef]
- Stojković, D.; Dias, M.I.; Drakulić, D.; Barros, L.; Stevanović, M.; Ferreira, I.C.F.R.; Soković, M.D. Methanolic extract of the herb Ononis spinosa L. Is an antifungal agent with no cytotoxicity to primary human cells. Pharmaceuticals 2020, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Banjanac, T.; Dragićević, M.; Šiler, B.; Gašić, U.; Bohanec, B.; Nestorović Živković, J.; Trifunović, S.; Mišić, D. Chemodiversity of two closely related tetraploid Centaurium species and their hexaploid hybrid: Metabolomic search for high-resolution taxonomic classifiers. Phytochemistry 2017, 140, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Robles-Escajeda, E.; Lerma, D.; Nyakeriga, A.M.; Ross, J.A.; Kirken, R.A.; Aguilera, R.J.; Varela-Ramirez, A. Searching in Mother Nature for Anti-Cancer Activity: Anti-Proliferative and Pro-Apoptotic Effect Elicited by Green Barley on Leukemia/Lymphoma Cells. PLoS ONE 2013, 8, e73508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura-Bencomo, S.; Gutierrez, D.A.; Robles-Escajeda, E.; Iglesias-Figueroa, B.; Siqueiros-Cendón, T.S.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Aguilera, R.J.; Rascón-Cruz, Q.; Varela-Ramirez, A. Recombinant human lactoferrin carrying humanized glycosylation exhibits antileukemia selective cytotoxicity, microfilament disruption, cell cycle arrest, and apoptosis activities. Investig. New Drugs 2020. [Google Scholar] [CrossRef]





| Peak No. | Identified Compounds | tR, min | Molecular Formula, [M–H]− | Calculated Mass, [M–H]− | Exact Mass, [M–H]− | Δ ppm | MS2 Fragments, (% Base Peak) | MS3 Fragments, (% Base Peak) | MS4 Fragments, (% Base Peak) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Acid Derivatives | ||||||||||
| 1 | Dihydroxybenzoyl hexoside d | 3.95 | C13H15O9− | 315.07216 | 315.07246 | −0.31 | 109(10), 152(22), 153(100), 154(9), 268(10), 278(9), 279(23) | 108(7), 109(100) | NA | / |
| 2 | Caffeoyl-hexoside isomer 1 c | 4.61 | C15H17O9− | 341.08781 | 341.08750 | 0.31 | 135(4), 179(100), 180(3) | 135(100) | 79(21), 107(100), 117(49) | [17] |
| 3 | Caffeic acid a,b | 4.61 | C9H7O4− | 179.03498 | 179.03487 | 0.11 | 89(19), 129(15), 134(11), 135(100), 143(23), 144(20), 161(13) | 91(9), 94(64), 106(6), 107(100), 132(5) | NA | [18] |
| 4 | Aesculin a,c | 4.88 | C15H15O9− | 339.07216 | 339.07142 | 0.74 | 177(100), 178(3) | 89(4), 105(10), 133(100), 149(6) | 89(100), 105(9), 123(15) | [17] |
| 5 | Caffeoyl-hexoside isomer 2 c | 5.11 | C15H17O9− | 341.08781 | 341.08674 | 1.07 | 135(9), 179(100), 180(7), 295(3) | 135(100) | 79(18), 107(100) | [17] |
| 6 | 5-O-Caffeoylquinic acid isomer 1 (Chlorogenic acid) a,b | 5.24 | C16H17O9− | 353.08781 | 353.08753 | 0.28 | 179(3), 191(100) | 85(95), 93(56), 111(41), 127(100), 171(32), 173(81) | 83(11), 85(100), 97(10), 99(38), 109(29) | [19] |
| 7 | p-Coumaric acid a,c | 5.29 | C9H7O3− | 163.04007 | 163.03968 | 0.39 | 99(13), 115(18), 116(12), 119(100), 128(16), 131(19), 135(12) | 66(100), 91(60) | NA | [17] |
| 8 | Ferulic acid a,c | 5.57 | C10H9O4− | 193.05063 | 193.05139 | −0.76 | 111(10), 134(6), 145(5), 147(100), 148(15), 149(9), 150(7) | 57(6), 85(13), 99(6), 101(7), 103(7), 119(7), 129(100) | 55(20), 57(50), 73(8), 85(100), 101(14) | [17] |
| 9 | 5-O-Caffeoylquinic acid isomer 2 b | 5.69 | C16H17O9− | 353.08781 | 353.08795 | −0.15 | 179(3), 191(100), 192(4) | 85(98), 93(58), 109(22), 111(31), 127(100), 173(69) | 81(4), 83(11), 85(100), 99(46), 109(27) | [19] |
| 10 | 5-O-p-Comaroylqunic acid c | 5.87 | C16H17O8− | 337.09289 | 337.09270 | 0.19 | 163(4), 173(8), 191(100) | 85(96), 93(64), 109(26), 111(35), 127(100), 173(91) | 81(17), 83(12), 85(100), 99(49), 109(59) | [20] |
| 11 | 5-O-Feruloylquinic acid isomer 1 c | 6.32 | C17H19O9− | 367.10346 | 367.10372 | −0.26 | 191(100), 192(8), 193(4), 321(4) | 85(100), 93(54), 109(25), 111(35), 127(93), 173(81) | 57(100) | [17] |
| 12 | 3,5-O-Dicaffeoylquinic acid c | 7.04 | C25H23O12− | 515.11950 | 515.11763 | 1.87 | 191(13), 335(9), 353(100), 354(14) | 179(4), 191(100) | 85(93), 93(63), 111(32), 127(100), 173(76) | [21] |
| 13 | Malonyl-1,4-O-dicaffeoylquinic acid c | 7.12 | C28H25O15− | 601.11989 | 601.12114 | −1.25 | 395(55), 439(72), 440(12), 515(85), 516(20), 557(100), 558(24) | 233(32), 335(4), 377(9), 395(100) | 173(13), 233(100), 335(8) | [22] |
| 14 | Malonyl-1,5-O-dicaffeoylquinic acid c | 7.34 | C28H25O15− | 601.11989 | 601.11856 | 1.33 | 233(10), 395(100), 396(13), 439(9), 515(5), 557(58), 558(10) | 173(12), 233(100), 335(5) | 155(3), 173(100) | [22] |
| 15 | Malonyl-4,5-O-dicaffeoylquinic acid c | 7.47 | C28H25O15− | 601.11989 | 601.12119 | −1.30 | 395(55), 396(11), 439(53), 515(56), 516(14), 557(100), 558(23) | 233(30), 335(4), 377(10), 395(100), 515(3) | 173(13), 233(100), 335(7) | [22] |
| 16 | 5-O-Feruloylquinic acid isomer 2 c | 7.79 | C17H19O9− | 367.10346 | 367.10270 | 0.75 | 191(100), 192(16), 321(17), 322(9), 323(9), 329(8), 330(13) | 85(99), 93(45), 109(29), 127(100), 171(26), 173(56) | NA | [17] |
| 17 | o-Hydroxybenzoic acid b | 8.17 | C7H5O3− | 137.02442 | 137.02435 | 0.06 | 93(100) | NA | [18] | |
| Flavonoid derivatives | ||||||||||
| 18 | Quercetin 3-O-(6″-rhamnosyl)-glucoside (Rutin) a,b | 6.40 | C27H29O16− | 609.14611 | 609.14539 | 0.72 | 225(5), 271(7), 300(37), 301(100), 343(12) | 151(77), 179(100), 255(45), 257(13), 271(76), 273(19) | 151(100) | [19] |
| 19 | Luteolin 7-O-glucoside (Cynaroside) a,b | 6.68 | C21H19O11− | 447.09329 | 447.08956 | 3.73 | 285(100), 286(13) | 151(41), 175(100), 199(87), 217(80), 241(97), 243(70) | 119(8), 131(86), 133(20), 147(100), 157(5) | [23] |
| 20 | Kaempferol 3-O-(6″-acetyl)-hexoside c | 7.20 | C23H21O12− | 489.10385 | 489.10298 | 0.87 | 285(100), 286(9), 429(6) | 151(37), 175(82), 199(88), 217(72), 241(100), 243(59) | 185(49), 197(99), 198(100), 199(79), 213(61) | [24] |
| 21 | Apigenin 7-O-glucoside (Apigetrin) a,c | 7.22 | C21H19O10− | 431.09837 | 431.09842 | −0.05 | 268(11), 269(100), 270(11), 311(3) | 149(30), 181(26), 183(27), 224(26), 225(100), 227(29) | 157(35), 169(44), 181(63), 196(40), 197(100) | [17] |
| 22 | Kaempferol 3-O-rhamnoside c | 7.65 | C21H19O10− | 431.09837 | 431.09750 | 0.87 | 255(6), 284(60), 285(100), 286(7), 327(4) | 229(51), 241(29), 255(58), 256(51), 257(100), 267(45) | 163(75), 185(14), 213(23), 229(100), 239(45) | [17] |
| 23 | Apigenin 7-O-(6″-acetyl)-hexoside c | 8.43 | C23H21O11− | 473.10894 | 473.10989 | −0.95 | 268(50), 269(100), 270(14), 311(6) | 149(30), 151(21), 183(28), 197(34), 201(24), 225(100) | 169(37), 181(57), 183(39), 196(21), 197(100) | [17] |
| 24 | Luteolin a,b | 8.65 | C15H9O6− | 285.04046 | 285.03874 | 1.73 | 151(28), 175(72), 197(21), 199(69), 217(55), 241(100), 243(53) | 197(100), 198(80), 199(59), 212(21), 213(44), 223(29) | 153(4), 169(100), 179(10), 180(14), 182(5) | [25] |
| 25 | Kaempferol 3-O-(6″-p-coumaroyl)-hexoside c | 9.39 | C30H25O13− | 593.13007 | 593.12989 | 0.18 | 285(100), 286(9), 307(31), 308(4) | 151(100), 213(50), 229(57), 241(42), 243(36), 257(87) | 83(4), 107(100) | [26] |
| 26 | Apigenin a,b | 9.50 | C15H9O5− | 269.04555 | 269.04531 | 0.23 | 149(45), 151(29), 183(17), 201(27), 225(100), 226(18), 227(18) | 169(13), 180(15), 181(100), 183(27), 196(20), 197(38) | 117(17), 139(25), 152(100), 153(41), 163(7) | [25] |
| 27 | Kaempferol 3-O-(4″-p-coumaroyl)-rhamnoside c | 9.90 | C30H25O12− | 577.13515 | 577.13701 | −1.86 | 285(100), 286(9) | 151(80), 229(38), 241(41), 255(33), 257(100), 267(27) | 163(27), 211(7), 213(16), 229(100), 239(12) | [27] |
| 28 | Isorhamnetin 3-O-(3″-p-coumaroyl)-rhamnoside d | 10.10 | C31H27O13− | 607.14572 | 607.14261 | 3.11 | 284(6), 285(27), 299(16), 300(17), 315(100), 316(11) | 300(100) | 151(21), 227(12), 255(61), 271(100), 272(51) | / |
| 29 | Quercetin 3-O-(2″,6″-di-p-coumaroyl)-hexoside c | 10.50 | C39H31O16− | 755.16176 | 755.16248 | −0.72 | 271(3), 285(4), 301(3), 307(6), 469(100), 470(20), 593(5) | 135(11), 161(56), 179(100), 271(22), 307(68) | NA | [28] |
| 30 | Kaempferol 3-O-(2″,6″-di-p-coumaroyl)-hexoside c | 11.08 | C39H31O15− | 739.16684 | 739.16671 | 0.14 | 285(9), 307(4), 453(100), 454(22), 455(4), 593(4) | 135(10), 161(100), 163(31), 179(65), 289(12), 307(67) | 117(3), 133(100) | [29] |
| 31 | Kaempferol 3-O-(2″,3″-di-p-coumaroyl)-rhamnoside c | 11.54 | C39H31O14− | 723.17193 | 723.16845 | 3.48 | 285(53), 286(8), 437(100), 438(17), 439(3), 577(3) | 145(100), 163(71), 187(24), 211(14), 273(29), 291(46) | 117(100) | [30] |
| 32 | Kaempferol 3-O-[2″-(4‴-methoxycinammoyl)-6″-p-coumaroyl]-hexoside c | 11.57 | C40H33O15− | 753.18249 | 753.18286 | −0.36 | 285(100), 286(9), 315(39), 437(49), 453(12), 467(77), 468(12) | 151(87), 185(47), 213(46), 229(60), 239(42), 257(100) | 189(28), 213(57), 215(13), 229(100), 239(24) | [29] |
| Cell lines | A. cerefolium IC50 (µg/mL) |
|---|---|
| A172 | 765.21 ± 56.7 |
| HGF-1 | >800 |
| Bacteria | A. cerefolium | Streptomycin | Ampicillin | |
|---|---|---|---|---|
| S. aureus (ATCC 11632) | MIC | 2.50 | 0.17 | 0.34 |
| MBC | 5.00 | 0.25 | 0.37 | |
| S. aureus MRSA | MIC | 1.25 | 0.10 | - |
| MBC | 2.50 | - | - | |
| Yeasts | A. cerefolium | Ketoconazole | Bifonazole | |
| C. albicans (ATCC 10231) | MIC | 1.25 | 0.50 | 0.15 |
| MFC | 1.25 | 1.00 | 0.30 | |
| C. krusei (clinical isolate) | MIC | 1.25 | 0.50 | 0.25 |
| MFC | 1.25 | 1.00 | 0.50 | |
| C. tropicalis (ATCC 750) | MIC | 0.62 | 0.30 | 0.25 |
| MFC | 1.25 | 0.50 | 0.50 |
| (A) Inhibition of S. aureus bacterial biofilm formation | |||||
|---|---|---|---|---|---|
| 1/2 MIC | 1/4 MIC | 1/8 MIC | 1/16 MIC | 1/32 MIC | |
| A. cerefolium | 69.88 ± 6.86 | 67.91 ± 4.99 | 44.25 ± 4.70 | NI | NI |
| Streptomycin | 55.64 ± 2.12 | 35.33 ± 1.47 | 33.22 ± 1.08 | 15.21 ± 1.12 | NI |
| (B) Inhibitiory and fungicidal effects on formed fungal biofilms | |||||
| Fungi | A. cerefolium | Fluconazole | |||
| MIC | MFC | MIC | MFC | ||
| C. albicans (ATCC 10231) | 5.00 | 10.00 | 8.00 | 9.00 | |
| C. krusei (clinical isolate) | 5.00 | 10.00 | 2.00 | 3.00 | |
| C. tropicalis (ATCC 750) | 5.00 | 10.00 | 3.00 | 6.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojković, D.; Drakulić, D.; Schwirtlich, M.; Rajčević, N.; Stevanović, M.; Soković, M.D.; Gašić, U. Extract of Herba Anthrisci cerefolii: Chemical Profiling and Insights into Its Anti-Glioblastoma and Antimicrobial Mechanism of Actions. Pharmaceuticals 2021, 14, 55. https://doi.org/10.3390/ph14010055
Stojković D, Drakulić D, Schwirtlich M, Rajčević N, Stevanović M, Soković MD, Gašić U. Extract of Herba Anthrisci cerefolii: Chemical Profiling and Insights into Its Anti-Glioblastoma and Antimicrobial Mechanism of Actions. Pharmaceuticals. 2021; 14(1):55. https://doi.org/10.3390/ph14010055
Chicago/Turabian StyleStojković, Dejan, Danijela Drakulić, Marija Schwirtlich, Nemanja Rajčević, Milena Stevanović, Marina D. Soković, and Uroš Gašić. 2021. "Extract of Herba Anthrisci cerefolii: Chemical Profiling and Insights into Its Anti-Glioblastoma and Antimicrobial Mechanism of Actions" Pharmaceuticals 14, no. 1: 55. https://doi.org/10.3390/ph14010055
APA StyleStojković, D., Drakulić, D., Schwirtlich, M., Rajčević, N., Stevanović, M., Soković, M. D., & Gašić, U. (2021). Extract of Herba Anthrisci cerefolii: Chemical Profiling and Insights into Its Anti-Glioblastoma and Antimicrobial Mechanism of Actions. Pharmaceuticals, 14(1), 55. https://doi.org/10.3390/ph14010055