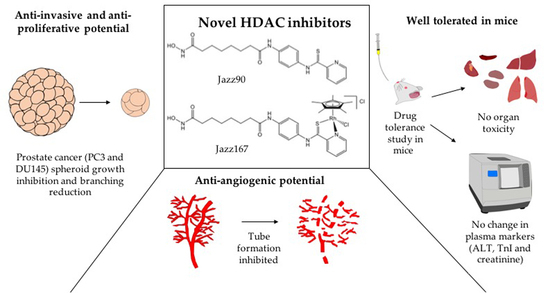
Anti-Proliferative, Anti-Angiogenic and Safety Profiles of Novel HDAC Inhibitors for the Treatment of Metastatic Castration-Resistant Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Compound Effects on Markers of Angiogenesis and EMT
2.2. Effects on Non-histone Mediated Pathways
2.3. Effects on 3D Spheroids
2.4. Safety and Tolerance of Jazz90 and Jazz167
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Maintenance
4.3. Western Blotting
4.4. Endothelial Tube Formation
4.5. Evaluation of Tumour Spheroids
4.6. Animal Housing and Care
4.7. Compound Administration
4.8. Organ and Blood Collection
4.9. TnI
4.10. Assessment of ALT Activity
4.11. Creatinine Levels
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, D.; Vashistha, V.; Isharwal, S.; Sediqe, S.A.; Lin, M.-F. Histone deacetylase inhibitors in castration-resistant prostate cancer: Molecular mechanism of action and recent clinical trials. Ther. Adv. Urol. 2015, 7, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, A.O. Progression of metastatic castrate-resistant prostate cancer: Impact of therapeutic intervention in the post-docetaxel space. J. Hematol. Oncol. 2011, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Jiang, X.; Liang, X.; Jiang, G. Molecular and cellular mechanisms of castration resistant prostate cancer (Review). Oncol. Lett. 2018, 15, 6063–6076. [Google Scholar] [CrossRef] [Green Version]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell. 2017, 32, 474–489.e6. [Google Scholar] [CrossRef] [Green Version]
- Cang, S.; Feng, J.; Konno, S.; Han, L.; Liu, K.; Sharma, S.C.; Choudhury, M.; Chiao, J.W. Deficient histone acetylation and excessive deacetylase activity as epigenomic marks of prostate cancer cells. Int. J. Oncol. 2009, 35, 1417–1422. [Google Scholar] [CrossRef] [Green Version]
- Rana, Z.; Diermeier, S.; Hanif, M.; Rosengren, R.J. Understanding Failure and Improving Treatment Using HDAC Inhibitors for Prostate Cancer. Biomedicines 2020, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA Approval Summary: Vorinostat for Treatment of Advanced Primary Cutaneous T-Cell Lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef]
- Bradley, D.; Rathkopf, D.; Dunn, R.; Stadler, W.M.; Liu, G.; Smith, D.C.; Pili, R.; Zwiebel, J.A.; Scher, H.I.; Hussain, M. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): Trial results and interleukin-6 analysis: A study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer 2009, 115, 5541–5549. [Google Scholar] [CrossRef] [Green Version]
- Ferrarelli, L.K. HDAC inhibitors in solid tumors and blood cancers. Sci. Signal. 2016, 9, ec216. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Picus, J.; Hussain, A.; Ellard, S.; Chi, K.N.; Nydam, T.; Allen-Freda, E.; Mishra, K.K.; Porro, M.G.; Scher, H.I.; et al. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2013, 72, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Molife, L.R.; Attard, G.; Fong, P.C.; Karavasilis, V.; Reid, A.H.M.; Patterson, S.; Riggs, C.E.; Higano, C.; Stadler, W.M.; McCulloch, W.; et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann. Oncol. 2010, 21, 109–113. [Google Scholar] [CrossRef]
- Eigl, B.J.; North, S.; Winquist, E.; Finch, D.; Wood, L.; Sridhar, S.S.; Powers, J.; Good, J.; Sharma, M.; Squire, J.A.; et al. A phase II study of the HDAC inhibitor SB939 in patients with castration resistant prostate cancer: NCIC clinical trials group study IND195. Invest. New Drugs 2015, 33, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Tsuboi, R.; Kato, Y.; Sugaya, M.; Tobinai, K.; Hamada, T.; Shimamoto, T.; Noguchi, K.; Iwatsuki, K. Phase I and pharmacokinetic study of the oral histone deacetylase inhibitor vorinostat in Japanese patients with relapsed or refractory cutaneous T-cell lymphoma: Phase I study of vorinostat in Japanese CTCL. J. Dermatol. 2012, 39, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Bates, S.E.; Wright, J.J.; Espinoza-Delgado, I.; Piekarz, R.L. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals 2010, 3, 2751–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.-F.; Zhong, W.-Z. Drug Design and Discovery: Principles and Applications. Molecules 2017, 22, 279. [Google Scholar] [CrossRef]
- Mahanta, N.; Szantai-Kis, D.M.; Petersson, E.J.; Mitchell, D.A. Biosynthesis and Chemical Applications of Thioamides. ACS Chem. Biol. 2019, 14, 142–163. [Google Scholar] [CrossRef]
- Nocentini, G.; Barzi, A. The 2,2′-Bipyridyl-6-carbothioamide copper (II) complex differs from the iron (II) complex in its biochemical effects in tumor cells, suggesting possible differences in the mechanism leading to cytotoxicity. Biochem. Pharmacol. 1996, 52, 65–71. [Google Scholar] [CrossRef]
- Verma, H.; Khatri, B.; Chakraborti, S.; Chatterjee, J. Increasing the bioactive space of peptide macrocycles by thioamide substitution. Chem. Sci. 2018, 9, 2443–2451. [Google Scholar] [CrossRef] [Green Version]
- Ndagi, U.; Mhlongo, N.; Soliman, M. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.-R.; Ke, Z.-F.; Tan, C.-P.; He, L.; Ji, L.-N.; Mao, Z.-W. Histone-Deacetylase-Targeted Fluorescent Ruthenium(II) Polypyridyl Complexes as Potent Anticancer Agents. Chem. Eur. J. 2013, 19, 10160–10169. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Arshad, J.; Astin, J.W.; Rana, Z.; Zafar, A.; Movassaghi, S.; Leung, E.; Patel, K.; Söhnel, T.; Reynisson, J.; et al. A Multitargeted Approach: Organorhodium Anticancer Agent Based on Vorinostat as a Potent Histone Deacetylase Inhibitor. Angew. Chem. Int. Ed. 2020, 132, 14717–14722. [Google Scholar] [CrossRef]
- Rana, Z.; Tyndall, J.D.A.; Hanif, M.; Hartinger, C.G.; Rosengren, R.J. Cytostatic Action of Novel Histone Deacetylase Inhibitors in Androgen Receptor-Null Prostate Cancer Cells. Pharmaceuticals 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Braicu, B.; Busuioc, D.; Gulei, R.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; Ionescu, C.; Berindan-Neagoe, I. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Xia, C.; Fang, J.; Rojanasakul, Y.; Jiang, B.-H. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006, 18, 2262–2271. [Google Scholar] [CrossRef]
- Butler, L.M.; Agus, D.B.; Scher, H.I.; Higgins, B.; Rose, A.; Cordon-Cardo, C.; Thaler, H.T.; Rifkind, R.A.; Marks, P.A.; Richon, V.M. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000, 60, 5165–5170. [Google Scholar] [PubMed]
- Bland, A.R.; Shrestha, N.; Bower, R.L.; Rosengren, R.J.; Ashton, J.C. The effect of metformin in EML4-ALK+ lung cancer alone and in combination with crizotinib in cell and rodent models. Biochem. Pharmacol. 2021, 183, 114345. [Google Scholar] [CrossRef]
- Shrestha, N.; Bland, A.R.; Bower, R.L.; Rosengren, R.J.; Ashton, J.C. Inhibition of Mitogen-Activated Protein Kinase Kinase Alone and in Combination with Anaplastic Lymphoma Kinase (ALK) Inhibition Suppresses Tumor Growth in a Mouse Model of ALK-Positive Lung Cancer. J. Pharmacol. Exp. Ther. 2020, 374, 134–140. [Google Scholar] [CrossRef]
- Damodaran, S.; Kyriakopoulos, C.E.; Jarrard, D.F. Newly Diagnosed Metastatic Prostate Cancer: Has the Paradigm Changed? Urol. Clin. North Am. 2017, 44, 611–621. [Google Scholar] [CrossRef]
- Huss, W.J.; Hanrahan, C.F.; Barrios, R.J.; Simons, J.W.; Greenberg, N.M. Angiogenesis and prostate cancer: Identification of a molecular progression switch. Cancer Res. 2001, 61, 2736–2743. [Google Scholar] [PubMed]
- Nordby, Y.; Andersen, S.; Richardsen, E.; Ness, N.; Al-Saad, S.; Melbø-Jørgensen, C.; Patel, H.R.H.; Dønnem, T.; Busund, L.; Bremnes, R.M. Stromal expression of VEGF-A and VEGFR-2 in prostate tissue is associated with biochemical and clinical recurrence after radical prostatectomy: Stromal Expression of VEGF-A and VEGFR-2 in Prostate. Prostate 2015, 75, 1682–1693. [Google Scholar] [CrossRef]
- Zhan, P.; Ji, Y.-N.; Yu, L.-K. VEGF is associated with the poor survival of patients with prostate cancer: A meta-analysis. Transl. Androl. Urol. 2013, 2, 99–105. [Google Scholar] [CrossRef]
- Peach, C.; Mignone, V.; Arruda, M.; Alcobia, D.; Hill, S.; Kilpatrick, L.; Woolard, J. Molecular pharmacology of VEGF-A isoforms: Binding and signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melegh, Z.; Oltean, S. Targeting Angiogenesis in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 2676. [Google Scholar] [CrossRef] [Green Version]
- Hrgovic, I.; Doll, M.; Pinter, A.; Kaufmann, R.; Kippenberger, S.; Meissner, M. Histone deacetylase inhibitors interfere with angiogenesis by decreasing endothelial VEGFR-2 protein half-life in part via a VE-cadherin-dependent mechanism. Exp. Dermatol. 2017, 26, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Bausch, D.; Knightly, T.; Liu, Z.; Li, Y.; Liu, B.; Lu, J.; Chong, W.; Velmahos, G.C.; Alam, H.B. Histone deacetylase inhibitors enhance endothelial cell sprouting angiogenesis in vitro. Surgery 2011, 150, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Urbich, C.; Rossig, L.; Kaluza, D.; Potente, M.; Boeckel, J.-N.; Knau, A.; Diehl, F.; Geng, J.; Hofmann, W.; Zeiher, A.M.; et al. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood 2009, 113, 5669–5679. [Google Scholar] [CrossRef] [Green Version]
- Turtoi, A.; Mottet, D.; Matheus, N.; Dumont, B.; Peixoto, P.; Hennequière, V.; Deroanne, C.; Colige, A.; Pauw, E.D.; Bellahcène, A.; et al. The angiogenesis suppressor gene AKAP12 is under the epigenetic control of HDAC7 in endothelial cells. Angiogenesis 2012, 15, 543–554. [Google Scholar] [CrossRef]
- Kaluza, D.; Kroll, J.; Gesierich, S.; Yao, T.-P.; Boon, R.A.; Hergenreider, E.; Tjwa, M.; Rössig, L.; Seto, E.; Augustin, H.G.; et al. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin: HDAC6 and angiogenesis. EMBO J. 2011, 30, 4142–4156. [Google Scholar] [CrossRef] [PubMed]
- Manickam, V.; Tiwari, A.; Jung, J.-J.; Bhattacharya, R.; Goel, A.; Mukhopadhyay, D.; Choudhury, A. Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi localized t-SNARE syntaxin 6. Blood 2011, 117, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.E.; Beswick, P. What does the aromatic ring number mean for drug design? Expert Opin. Drug Discov. 2014, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Härmä, V.; Virtanen, J.; Mäkelä, R.; Happonen, A.; Mpindi, J.-P.; Knuuttila, M.; Kohonen, P.; Lötjönen, J.; Kallioniemi, O.; Nees, M. A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PLoS ONE 2010, 5, e10431. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, J.; Hammer, M.; Muhs, S.; Kohn, M.; Pryalukhin, A.; Veith, C.; Bohle, R.M.; Stöckle, M.; Junker, K.; Saar, M. Patient-derived, three-dimensional spheroid cultures provide a versatile translational model for the study of organ-confined prostate cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 551–559. [Google Scholar] [CrossRef]
- Lobjois, V.; Frongia, C.; Jozan, S.; Truchet, I.; Valette, A. Cell cycle and apoptotic effects of SAHA are regulated by the cellular microenvironment in HCT116 multicellular tumour spheroids. Eur. J. Cancer 2009, 45, 2402–2411. [Google Scholar] [CrossRef]
- Gupta, S.; Pungsrinont, T.; Ženata, O.; Neubert, L.; Vrzal, R.; Baniahmad, A. Interleukin-23 Represses the Level of Cell Senescence Induced by the Androgen Receptor Antagonists Enzalutamide and Darolutamide in Castration-Resistant Prostate Cancer Cells. Horm Cancer 2020, 11, 182–190. [Google Scholar] [CrossRef]
- Perše, M.; Večerić-Haler, Ž. Cisplatin-Induced Rodent Model of Kidney Injury: Characteristics and Challenges. BioMed Res. Int. 2018, 2018, 1–29. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bakhaat, G.A.; Tammam, H.G.; Mohamed, R.M.; El-Naggar, S.A. Cardioprotective effect of green tea extract and vitamin E on Cisplatin-induced cardiotoxicity in mice: Toxicological, histological and immunohistochemical studies. Biomed Pharmacother. 2019, 113, 108731. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Zhou, S.; Zhang, X.; Bo, H. The antitumor effect and toxicity of a ruthenium(II) complex in vivo. Inorg. Chem. Commun. 2018, 87, 49–52. [Google Scholar] [CrossRef]
- Peixoto, R.C.A.; Miranda-Vilela, A.L.; Filho, J.d.S.; Carneiro, M.L.B.; Oliveira, R.G.S.; da Silva, M.O.; de Souza, A.R.; Báo, S.N. Antitumor effect of free rhodium (II) citrate and rhodium (II) citrate-loaded maghemite nanoparticles on mice bearing breast cancer: A systemic toxicity assay. Tumor Biol. 2015, 36, 3325–3336. [Google Scholar] [CrossRef] [Green Version]
- Threatt, S.D.; Synold, T.W.; Wu, J.; Barton, J.K. In vivo anticancer activity of a rhodium metalloinsertor in the HCT116 xenograft tumor model. Proc. Natl. Acad. Sci. USA 2020, 117, 17535–17542. [Google Scholar] [CrossRef]
- Hiriyan, J.; Shivarudraiah, P.; Gavara, G.; Annamalai, P.; Natesan, S.; Sambasivam, G.; Sukumaran, S.K. Discovery of PAT-1102, a novel, potent and orally active histone deacetylase inhibitor with antitumor activity in cancer mouse models. Anticancer Res. 2015, 35, 229–237. [Google Scholar] [PubMed]
- Kerr, J.S.; Galloway, S.; Lagrutta, A.; Armstrong, M.; Miller, T.; Richon, V.M.; Andrews, P.A. Nonclinical Safety Assessment of the Histone Deacetylase Inhibitor Vorinostat. Int. J. Toxicol. 2010, 29, 3–19. [Google Scholar] [CrossRef]
- Dovey, O.M.; Foster, C.T.; Conte, N.; Edwards, S.A.; Edwards, J.M.; Singh, R.; Vassiliou, G.; Bradley, A.; Cowley, S.M. Histone deacetylase 1 and 2 are essential for normal T-cell development and genomic stability in mice. Blood 2013, 121, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Zhou, Y.; Ji, H.; Wang, Y.; Wu, Q.; Bao, J.; Ye, F.; Shi, Y.; Bu, H. Loss of histone deacetylases 1 and 2 in hepatocytes impairs murine liver regeneration through Ki67 depletion: Hepatology. Hepatology 2013, 58, 2089–2098. [Google Scholar] [CrossRef]
- Grausenburger, R.; Bilic, I.; Boucheron, N.; Zupkovitz, G.; El-Housseiny, L.; Tschismarov, R.; Zhang, Y.; Rembold, M.; Gaisberger, M.; Hartl, A.; et al. Conditional Deletion of Histone Deacetylase 1 in T Cells Leads to Enhanced Airway Inflammation and Increased Th2 Cytokine Production. J. Immunol. 2010, 185, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; de Bono, J.; Deangelo, D.J.; van Oosterom, A.; Morganroth, J.; Laird, G.H.; Dugan, M.; Scott, J.W.; Ottmann, O.G. Cardiac monitoring in phase I trials of a novel histone deacetylase (HDAC) inhibitor LAQ824 in patients with advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2005, 23 (Suppl. 16), 3131. [Google Scholar] [CrossRef]
- Sager, P.T.; Balser, B.; Wolfson, J.; Nichols, J.; Pilot, R.; Jones, S.; Burris, H.A. Electrocardiographic effects of class 1 selective histone deacetylase inhibitor romidepsin. Cancer Med. 2015, 4, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Deroanne, C.F.; Bonjean, K.; Servotte, S.; Devy, L.; Colige, A.; Clausse, N.; Blacher, S.; Verdin, E.; Foidart, J.; Nusgens, B.V.; et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene 2002, 21, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.-C.; Chang, W.-C.; Hsu, T.-I.; Liu, J.-J.; Yeh, S.-H.; Wang, J.-Y.; Liou, J.-P.; Ko, C.-Y.; Chang, K.-Y.; Chuang, J.-Y. Suberoylanilide hydroxamic acid represses glioma stem-like cells. J. Biomed. Sci. 2016, 23, 81. [Google Scholar] [CrossRef] [Green Version]
- Robertson, F.M.; Ogasawara, M.A.; Ye, Z.; Chu, K.; Pickei, R.; Debeb, B.G.; Woodward, W.A.; Hittelman, W.N.; Cristofanilli, M.; Barsky, S.H. Imaging and Analysis of 3D Tumor Spheroids Enriched for a Cancer Stem Cell Phenotype. J. Biomol. Screen. 2010, 15, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Giles, F.; Fischer, T.; Cortes, J.; Garcia-Manero, G.; Beck, J.; Ravandi, F.; Masson, E.; Rae, P.; Laird, G.; Sharma, S.; et al. A Phase I Study of Intravenous LBH589, a Novel Cinnamic Hydroxamic Acid Analogue Histone Deacetylase Inhibitor, in Patients with Refractory Hematologic Malignancies. Clin. Cancer Res. 2006, 12, 4628–4635. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Treatment | Dose (mg/kg) | Heart | Spleen | Lungs | Liver | Testes | Kidney | Brain |
|---|---|---|---|---|---|---|---|---|
| Jazz90 | 25 | 0.63 ± 0.03 | 0.29 ± 0.02 | 1.22 ± 0.08 | 5.31 ± 0.2 | 0.72 ± 0.03 | 1.83 ± 0.11 | 1.16 ± 0.06 |
| 50 | 0.65 ± 0.10 | 0.30 ± 0.02 | 1.04 ± 0.17 | 5.36 ± 0.2 | 0.65 ± 0.03 | 1.72 ± 0.10 | 1.15 ± 0.07 | |
| 75 | 0.66 ± 0.02 | 0.29 ± 0.03 | 1.21 ± 0.04 | 4.93 ± 0.1 | 0.64 ± 0.05 | 1.84 ± 0.07 | 1.25 ± 0.08 | |
| Jazz167 | 25 | 0.55 ± 0.02 | 0.28 ± 0.01 | 0.79 ± 0.07 | 5.35 ± 0.3 | 0.69 ± 0.08 | 1.73 ± 0.07 | 1.38 ± 0.09 |
| 50 | 0.55 ± 0.02 | 0.29 ± 0.02 | 0.92 ± 0.12 | 5.26 ± 0.2 | 0.67 ± 0.01 | 1.78 ± 0.04 | 1.39 ± 0.09 | |
| 75 | 0.60 ± 0.05 | 0.28 ± 0.01 | 1.11 ± 0.23 | 5.01 ± 0.2 | 0.65 ± 0.04 | 1.72 ± 0.12 | 1.38 ± 0.09 | |
| Vehicle (5% DMSO) | 0.56 ± 0.01 | 0.27 ± 0.01 | 0.89 ± 0.08 | 5.10 ± 0.1 | 0.65 ± 0.02 | 1.82 ± 0.07 | 1.39 ± 0.10 | |
| Untreated | 0.65 ± 0.10 | 0.30 ± 0.01 | 1.07 ± 0.15 | 4.81 ± 0.1 | 0.68 ± 0.02 | 1.84 ± 0.03 | 1.16 ± 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, Z.; Diermeier, S.; Walsh, F.P.; Hanif, M.; Hartinger, C.G.; Rosengren, R.J. Anti-Proliferative, Anti-Angiogenic and Safety Profiles of Novel HDAC Inhibitors for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Pharmaceuticals 2021, 14, 1020. https://doi.org/10.3390/ph14101020
Rana Z, Diermeier S, Walsh FP, Hanif M, Hartinger CG, Rosengren RJ. Anti-Proliferative, Anti-Angiogenic and Safety Profiles of Novel HDAC Inhibitors for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Pharmaceuticals. 2021; 14(10):1020. https://doi.org/10.3390/ph14101020
Chicago/Turabian StyleRana, Zohaib, Sarah Diermeier, Fearghal P. Walsh, Muhammad Hanif, Christian G. Hartinger, and Rhonda J. Rosengren. 2021. "Anti-Proliferative, Anti-Angiogenic and Safety Profiles of Novel HDAC Inhibitors for the Treatment of Metastatic Castration-Resistant Prostate Cancer" Pharmaceuticals 14, no. 10: 1020. https://doi.org/10.3390/ph14101020
APA StyleRana, Z., Diermeier, S., Walsh, F. P., Hanif, M., Hartinger, C. G., & Rosengren, R. J. (2021). Anti-Proliferative, Anti-Angiogenic and Safety Profiles of Novel HDAC Inhibitors for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Pharmaceuticals, 14(10), 1020. https://doi.org/10.3390/ph14101020