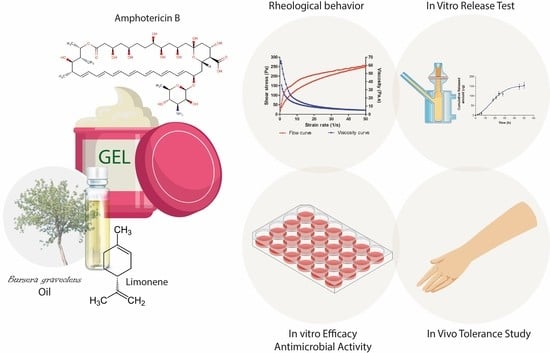
Development of a Topical Amphotericin B and Bursera graveolens Essential Oil-Loaded Gel for the Treatment of Dermal Candidiasis
Abstract
:1. Introduction
2. Results
2.1. Solubility Studies
2.2. Design and Preparation of AmB + BGEO Gel Formulation
2.3. Characterization of AmB + BGEO Gel
2.4. Stability Studies
2.5. In Vitro Release Study
2.6. Ex Vivo Permeation Study
2.7. Efficacy Study: Antimicrobial Activity
2.8. Tolerance Studies
2.8.1. Cytotoxicity Studies by MTT Assay
2.8.2. In Vivo Tolerance Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. High-Performance Liquid Chromatography (HPLC)
4.3. Solubility Studies
4.4. Formulation: Design and Analysis of 23 Factorial Experiment
4.5. Characterization of AmB + BGEO Gel
4.6. Stability Studies
4.7. In Vitro Release Study
4.8. Ex Vivo Permeation Study
4.9. Efficacy Study: Antimicrobial Activity
4.10. Tolerance Studies
4.10.1. Cytotoxicity Studies by MTT Method
4.10.2. In Vivo Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, C.J.; Wagner, D.K.; Sohnle, P.G. Fungal Infections, Cutaneous. In Encyclopedia of Microbiology, 3rd ed.; Elsevier: New York, NY, USA, 2009; pp. 382–388. [Google Scholar]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef]
- Permana, A.D.; Paredes, A.J.; Volpe-Zanutto, F.; Anjani, Q.K.; Utomo, E.; Donnelly, R.F. Dissolving microneedle-mediated dermal delivery of itraconazole nanocrystals for improved treatment of cutaneous candidiasis. Eur. J. Pharm. Biopharm. 2020, 154, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Palese, E.; Nudo, M.; Zino, G.; Devirgiliis, V.; Carbotti, M.; Cinelli, E.; Rodio, D.M.; Bressan, A.; Prezioso, C.; Ambrosi, C.; et al. Cutaneous candidiasis caused by Candida albicans in a young non-immunosuppressed patient: An unusual presentation. Int. J. Immunopathol. Pharm. 2018, 32, 2058738418781368. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Rex, J.H.; Sobel, J.D.; Filler, S.G.; Dismukes, W.E.; Walsh, T.J.; Edwards, J.E. Guidelines for Treatment of Candidiasis. Clin. Infect. Dis. 2004, 38, 161–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taudorf, E.H.; Jemec, G.B.E.; Hay, R.J.; Saunte, D.M.L. Cutaneous candidiasis—An evidence-based review of topical and systemic treatments to inform clinical practice. J. Eur. Acad. Derm. Venereol. 2019, 33, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Camp, W.L.; Elewski, B.E. Advances in topical and systemic antifungals. Derm. Clin. 2007, 25, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Morio, F.; Jensen, R.H.; Le Pape, P.; Arendrup, M.C. Molecular basis of antifungal drug resistance in yeasts. Int. J. Antimicrob. Agents 2017, 50, 599–606. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Tanabe, K.; Niimi, M.; Monk, B.C. Candida albicans drug resistance another way to cope with stress. Microbiology 2007, 153, 3211–3217. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes-Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Yamamoto, T.; Umegawa, Y.; Tsuchikawa, H.; Hanashima, S.; Matsumori, N.; Funahashi, K.; Seo, S.; Shinoda, W.; Murata, M. The Amphotericin B-Ergosterol Complex Spans a Lipid Bilayer as a Single-Length Assembly. Biochemistry 2019, 58, 5188–5196. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Preuss, C.V. Antifungal Membrane Function Inhibitors (Amphotericin B); StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New Facets of Antifungal Therapy. Virulence 2016, 8, 222–236. [Google Scholar] [CrossRef] [Green Version]
- Ito, J.I.; Hooshmand-Rad, R. Treatment of Candida Infections with Amphotericin B Lipid Complex. Clin. Infect. Dis. 2005, 40, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Laniado-Laborin, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, R.; de Pablo, E.; Ballesteros, M.P.; Serrano, D.R. Unmet clinical needs in the treatment of systemic fungal infections: The role of amphotericin B and drug targeting. Int. J. Pharm. 2017, 525, 139–148. [Google Scholar] [CrossRef]
- Lopez-Castillo, C.; Rodriguez-Fernandez, C.; Cordoba, M.; Torrado, J.J. Permeability Characteristics of a New Antifungal Topical Amphotericin B Formulation with gamma-Cyclodextrins. Molecules 2018, 23, 3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa, L.; Calpena, A.C.; Silva-Abreu, M.; Espinoza, L.C.; Rincon, M.; Bozal, N.; Domenech, O.; Rodriguez-Lagunas, M.J.; Clares, B. Thermoreversible Gel-Loaded Amphotericin B for the Treatment of Dermal and Vaginal Candidiasis. Pharmaceutics 2019, 11, 312. [Google Scholar] [CrossRef] [Green Version]
- Carvajal-Vidal, P.; Mallandrich, M.; Garcia, M.L.; Calpena, A.C. Effect of Different Skin Penetration Promoters in Halobetasol Propionate Permeation and Retention in Human Skin. Int. J. Mol. Sci. 2017, 18, 2475. [Google Scholar] [CrossRef] [Green Version]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design Principles of Chemical Penetration Enhancers for Transdermal Drug Delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Jiang, Q.D.; Wu, Y.M.; Liu, P.; Yao, J.H.; Lu, Q.; Zhang, H.; Duan, J.A. Potential of Essential Oils as Penetration Enhancers for Transdermal Administration of Ibuprofen to Treat Dysmenorrhoea. Molecules 2015, 20, 18219–18236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Monzotea, L.; Hillb, G.; Cuellarc, A.; Scullc, R.; Setzerb, W. Chemical Composition and Anti-proliferative Properties of Bursera graveolens Essential Oil. Nat. Prod. Commun. 2012, 7, 1531–1534. [Google Scholar] [CrossRef] [Green Version]
- Rey-Valeirón, C.; Guzmán, L.; Saa, L.R.; López-Vargas, J.; Valarezo, E. Acaricidal activity of essential oils of Bursera graveolens (Kunth) Triana & Planch and Schinus molle L. on unengorged larvae of cattle tick Rhipicephalus (Boophilus) microplus (Acari:Ixodidae). J. Essent. Oil Res. 2017, 29, 344–350. [Google Scholar] [CrossRef]
- Carrión-Paladines, V.; Fries, A.; Caballero, R.E.; Pérez Daniëls, P.; García-Ruiz, R. Biodegradation of Residues from the Palo Santo (Bursera graveolens) Essential Oil Extraction and Their Potential for Enzyme Production Using Native Xylaria Fungi from Southern Ecuador. Fermentation 2019, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Young, D.G.; Chao, S.; Casablanca, H.; Bertrand, M.-C.; Minga, D. Essential Oil of Bursera graveolens (Kunth) Triana et Planch from Ecuador. J. Essent. Oil Res. 2007, 19, 525–526. [Google Scholar] [CrossRef]
- Tutaj, K.; Szlazak, R.; Szalapata, K.; Starzyk, J.; Luchowski, R.; Grudzinski, W.; Osinska-Jaroszuk, M.; Jarosz-Wilkolazka, A.; Szuster-Ciesielska, A.; Gruszecki, W.I. Amphotericin B-silver hybrid nanoparticles: Synthesis, properties and antifungal activity. Nanomedicine 2016, 12, 1095–1103. [Google Scholar] [CrossRef]
- Welin-Berger, K.; Neelissen, J.; Bergenstahl, B. In vitro permeation profile of a local anaesthetic compound from topical formulations with different rheological behaviour—Verified by in vivo efficacy data. Eur. J. Pharm. Sci. 2001, 14, 229–236. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Silva-Abreu, M.; Calpena, A.C.; Rodriguez-Lagunas, M.J.; Fabrega, M.J.; Garduno-Ramirez, M.L.; Clares, B. Nanoemulsion strategy of pioglitazone for the treatment of skin inflammatory diseases. Nanomedicine 2019, 19, 115–125. [Google Scholar] [CrossRef]
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Vera-Garcia, R.; Silva-Abreu, M.; Domenech, O.; Badia, J.; Rodriguez-Lagunas, M.J.; Clares, B.; Calpena, A.C. Topical Pioglitazone Nanoformulation for the Treatment of Atopic Dermatitis: Design, Characterization and Efficacy in Hairless Mouse Model. Pharmaceutics 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Suner-Carbo, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.J.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montanes, A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Berenguer, D.; Alcover, M.M.; Sessa, M.; Halbaut, L.; Guillen, C.; Boix-Montanes, A.; Fisa, R.; Calpena-Campmany, A.C.; Riera, C.; Sosa, L. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics 2020, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Asghar, L.F.; Chandran, S. Design and evaluation of matrix base with sigmoidal release profile for colon-specific delivery using a combination of Eudragit and non-ionic cellulose ether polymers. Drug Deliv. Transl. Res. 2011, 1, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Hagen, M.; Baker, M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr. Med. Res. Opin. 2017, 33, 1623–1634. [Google Scholar] [CrossRef] [Green Version]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.C.; Chiang, B.H.; Huang, D.W.; Li, P.H. Skin permeation of D-limonene-based nanoemulsions as a transdermal carrier prepared by ultrasonic emulsification. Ultrason. Sonochem. 2014, 21, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Teng, Y.; Wang, H.; Hou, H. Enhancement of skin permeation of bufalin by limonene via reservoir type transdermal patch: Formulation design and biopharmaceutical evaluation. Int. J. Pharm. 2013, 447, 231–240. [Google Scholar] [CrossRef]
- Lim, P.F.; Liu, X.Y.; Kang, L.; Ho, P.C.; Chan, Y.W.; Chan, S.Y. Limonene GP1/PG organogel as a vehicle in transdermal delivery of haloperidol. Int. J. Pharm. 2006, 311, 157–164. [Google Scholar] [CrossRef]
- Mendez, A.H.S.; Cornejo, C.G.F.; Coral, M.F.C.; Arnedo, M.C.A. Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oil of Bursera graveolens (Burseraceae) From Perú. Indian J. Pharm. Educ. Res. 2017, 51, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.E.; Lai, K.S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [Green Version]
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnopharmacol. 2020, 262, 113135. [Google Scholar] [CrossRef]
- Muslim, S.N.; Hussin, Z.S. Chemical compounds and synergistic antifungal properties of Thymus kotschanus essential oil plus ketoconazole against Candida spp. Gene Rep. 2020, 21, 100916. [Google Scholar] [CrossRef]
- Mahboubi, M.; Ghazian Bidgoli, F. In vitro synergistic efficacy of combination of amphotericin B with Myrtus communis essential oil against clinical isolates of Candida albicans. Phytomedicine 2010, 17, 771–774. [Google Scholar] [CrossRef]
- Scherliess, R. The MTT assay as tool to evaluate and compare excipient toxicity in vitro on respiratory epithelial cells. Int. J. Pharm. 2011, 411, 98–105. [Google Scholar] [CrossRef]
- Sarango-Granda, P.; Silva-Abreu, M.; Calpena, A.C.; Halbaut, L.; Fabrega, M.J.; Rodriguez-Lagunas, M.J.; Diaz-Garrido, N.; Badia, J.; Espinoza, L.C. Apremilast Microemulsion as Topical Therapy for Local Inflammation: Design, Characterization and Efficacy Evaluation. Pharmaceuticals 2020, 13, 484. [Google Scholar] [CrossRef]
- Sosa, L.; Clares, B.; Alvarado, H.L.; Bozal, N.; Domenech, O.; Calpena, A.C. Amphotericin B releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomedicine 2017, 13, 2303–2312. [Google Scholar] [CrossRef]
- Costa, G.M.D.A.; Alves, G.d.A.D.; Maia Campos, P.M.B.G. Application of design of experiments in the development of cosmetic formulation based on natural ingredients. Int. J. Phytocosmetics Nat. Ingred. 2019, 6, 4. [Google Scholar] [CrossRef]
- Rowe, R.; Sheskey, P.; Owen, S. Handbook of Pharmaceutical Excipients, 5th ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Churchill Livingstone Elsevier: London, UK, 2014. [Google Scholar]
- Campana-Seoane, M.; Peleteiro, A.; Laguna, R.; Otero-Espinar, F.J. Bioadhesive emulsions for control release of progesterone resistant to vaginal fluids clearance. Int. J. Pharm. 2014, 477, 495–505. [Google Scholar] [CrossRef] [PubMed]
- ICH. Stability Testing of New Drug Substances and Products Q1A(R2). In International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; ICH: Geneva, Switzerland, 2003. [Google Scholar]
- Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST Definitive Document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute, CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, 3rd ed.; Document M27-A3; Clinical and Laboratory Standards Institute: Villanova, PA, USA, 2008; Volume 28, ISBN 1-56238-666-2. [Google Scholar]






| 1 Day | 60 Days | |||||
|---|---|---|---|---|---|---|
| Formulation | Appearance | pH | Drug Content (%) | Appearance | pH | Drug Content (%) |
| F1 | Homogeneous | 4.80 ± 0.03 | 99.68 ± 0.16 | Precipitates | 4.12 ± 0.09 | 96.78 ± 1.67 |
| F2 | Homogeneous | 6.76 ± 0.05 | 99.73 ± 0.12 | Homogeneous | 4.98 ± 0.07 | 98.25 ± 0.82 |
| F3 | Homogeneous | 5.85 ± 0.09 | 99.65 ± 0.08 | Homogeneous | 5.57 ± 0.04 | 99.18 ± 0.43 |
| F4 | Homogeneous | 6.81 ± 0.07 | 99.66 ± 0.10 | Lumps | 6.02 ± 0.10 | 98.86 ± 1.35 |
| F5 | Homogeneous | 5.28 ± 0.01 | 99.52 ± 0.09 | Homogeneous | 4.16 ± 0.15 | 98.16 ± 0.98 |
| F6 | Homogeneous | 7.12 ± 0.08 | 99.59 ± 0.13 | Precipitates | 6.15 ± 0.09 | 97.63 ± 1.82 |
| F7 | Homogeneous | 5.92 ± 0.04 | 99.81 ± 0.09 | Homogeneous | 5.05 ± 0.07 | 98.83 ± 0.66 |
| F8 | Homogeneous | 6.70 ± 0.06 | 99.65 ± 0.11 | Precipitates | 5.72 ± 0.25 | 97.15 ± 1.68 |
| Component (%) | |
|---|---|
| Amphotericin B (AmB) | 0.1 |
| B. graveolens essential oil (BGEO) | 2 |
| Carboxymethylcelullose (CMC) | 3 |
| Propylene glycol 400 | 20 |
| Sodium benzoate | 0.02 |
| Citric acid | 0.2 |
| Dimethylsulfoxyde (DMSO) | 5 |
| Water | 69.68 |
| Time (Days) | 30 ± 2 °C/65 ± 5% RH | 40 ± 2 °C/75 ± 5% RH | ||||
|---|---|---|---|---|---|---|
| Appearance | pH | Drug Content (%) | Appearance | pH | Drug Content (%) | |
| 1 | Homogeneous | 5.85 ± 0.09 | 99.65 ± 0.08 | Homogeneous | 5.83 ± 0.07 | 99.67 ± 0.12 |
| 60 | Homogeneous | 5.57 ± 0.04 | 99.18 ± 0.43 | Homogeneous | 5.43 ± 0.15 | 98.91 ± 0.17 |
| 120 days | Homogeneous | 5.48 ± 0.12 | 98.79 ± 0.66 | Homogeneous | 5.12 ± 0.07 | 98.56 ± 0.32 |
| Tested Species | Origin | MIC (µg/mL) | % (v/v) | ||
|---|---|---|---|---|---|
| Free AmB | AmB Gel | AmB + BGEO Gel | BGEO | ||
| C. albicans | ATCC 10231 | 0.15 | 0.58 | 0.29 | 12.50 |
| C. grabrata | ATCC 66032 | 0.60 | 0.58 | 0.39 | 6.25 |
| C. parapsilosis | ATCC 22019 | 0.30 | 1 | 0.58 | 12.50 |
| Factors | Levels | |
|---|---|---|
| (+) | (−) | |
| A: Polymer | Carboxymethylcellulose | Carbopol |
| B: Cosolvent | Glycerin | Propylene glycol 400 |
| C: Preservative | Sodium benzoate | Parabens |
| Component | % | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|---|
| CMC | 3 | * | * | * | * | ||||
| Carbopol 940 | 1 | * | * | * | * | ||||
| Glycerin | 20 | * | * | * | * | ||||
| Propylene glycol 400 | 20 | * | * | * | * | ||||
| Sodium benzoate | 0.02 | * | * | * | * | ||||
| Parabens | 0.02 | * | * | * | * | ||||
| Citric acid | 0.20 | * | * | * | * | ||||
| Triethanolamine | 1 | * | * | * | * | ||||
| AmB | 0.1 | * | * | * | * | * | * | * | * |
| DMSO | 5 | * | * | * | * | * | * | * | * |
| BGEO | 2 | * | * | * | * | * | * | * | * |
| Water | sq | * | * | * | * | * | * | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, L.C.; Sosa, L.; Granda, P.C.; Bozal, N.; Díaz-Garrido, N.; Chulca-Torres, B.; Calpena, A.C. Development of a Topical Amphotericin B and Bursera graveolens Essential Oil-Loaded Gel for the Treatment of Dermal Candidiasis. Pharmaceuticals 2021, 14, 1033. https://doi.org/10.3390/ph14101033
Espinoza LC, Sosa L, Granda PC, Bozal N, Díaz-Garrido N, Chulca-Torres B, Calpena AC. Development of a Topical Amphotericin B and Bursera graveolens Essential Oil-Loaded Gel for the Treatment of Dermal Candidiasis. Pharmaceuticals. 2021; 14(10):1033. https://doi.org/10.3390/ph14101033
Chicago/Turabian StyleEspinoza, Lupe Carolina, Lilian Sosa, Paulo C. Granda, Nuria Bozal, Natalia Díaz-Garrido, Brenda Chulca-Torres, and Ana Cristina Calpena. 2021. "Development of a Topical Amphotericin B and Bursera graveolens Essential Oil-Loaded Gel for the Treatment of Dermal Candidiasis" Pharmaceuticals 14, no. 10: 1033. https://doi.org/10.3390/ph14101033
APA StyleEspinoza, L. C., Sosa, L., Granda, P. C., Bozal, N., Díaz-Garrido, N., Chulca-Torres, B., & Calpena, A. C. (2021). Development of a Topical Amphotericin B and Bursera graveolens Essential Oil-Loaded Gel for the Treatment of Dermal Candidiasis. Pharmaceuticals, 14(10), 1033. https://doi.org/10.3390/ph14101033