Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders
Abstract
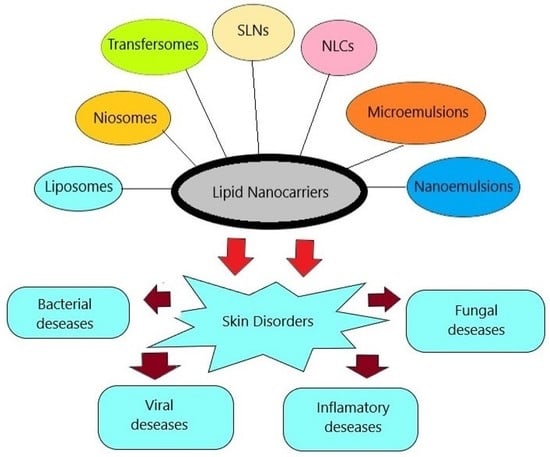
:1. Introduction
2. Skin
3. Skin Disorders
Treatment of Skin Disorders with Conventional Topical Delivery Systems
4. Lipid-Based Drug Delivery Systems
5. Nanovesicular Carriers
5.1. Emerging Lipid Nanovesicular Carriers
5.2. Nanovesicular Carriers in the Treatment of Skin Disorders
5.2.1. Antipsoriatic Effect
5.2.2. Antifungal Effect
5.2.3. Anti-Vitiligo Effect
5.2.4. Anti-Acne Effect
- The proliferation of propionibacterium acnes bacteria in pilosebaceous units of the skin;
- Local inflammation [135].
5.2.5. Antiviral Effect
5.2.6. Local Anesthetic Effect
5.2.7. Antibiotic Effect
5.2.8. Anticarcinogenic Effect
6. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
6.1. Solid Lipid Nanoparticles
6.2. Nanostructured Lipid Carriers
6.3. Preparation of SLN and NLC
- Hot homogenization—the lipids are heated above their melting point;
6.4. SLN and NLC in the Treatment of Skin Disorders
6.4.1. Antioxidant Effect
6.4.2. Anti-Inflammatory Effect
6.4.3. Antifungal Effect
6.4.4. Anti-Acne Effect
7. Microemulsions and Nanoemulsions
7.1. Microemulsions
- Oil-in-water (O/W) microemulsion;
- Water-in-oil (W/O) microemulsion;
- Bicontinuous microemulsion [232].
7.2. Nanoemulsions
7.3. Microemulsions and Nanoemulsions in the Treatment of Skin Disorders
7.3.1. Antipsoriatic Effect
7.3.2. Antifungal Effect
7.3.3. Anti-Inflammatory Effect
7.3.4. Antioxidant Effect
7.3.5. Local Anesthetic Effect
7.3.6. Anticarcinogenic Effect
8. Topical Dosage Forms with Lipid Nanoparticulate DDS for the Treatment of Skin Disorders
9. Future Prospects of Lipid Nanoparticulate DDS for the Treatment of Skin Disorders
- Precise delivery across the skin and to certain skin strata, depending on the final target;
- Successful elimination of lipid nanomaterial toxicity threats in topical medical formulations and cosmetics;
- Ensuring improved permeation and low skin irritability as a result of the use of lipid nanocarriers;
- Improved cutaneous release of incorporated API with a broad spectrum of physiological and physicochemical properties.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanclemente, G.; Burgos, C.; Nova, J.; Hernández, F.; González, C.; Reyes, M.I.; Córdoba, N.; Arévalo, Á.; Meléndez, E.; Colmenares, J.; et al. The impact of skin diseases on quality of life: A multicenter study. Actas Dermosifiliogr. 2017, 108, 244–252, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.F. Global burden of skin disease: Inequities and innovations. Curr. Dermatol. Rep. 2017, 6, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Barambhe, M.S.; Jain, J.; Jajoo, U.N.; Pandey, N. Prevalence of skin diseases in rural Central India: A community-based, cross-sectional, observational study. J. Mahatma Gandhi Inst. Med. Sci. 2016, 21, 111–115. [Google Scholar] [CrossRef]
- Yew, Y.W.; Kuan, A.H.Y.; Ge, L.; Yap, C.W.; Heng, B.H. Psychosocial impact of skin diseases: A population-based study. PLoS ONE 2020, 15, e0244765. [Google Scholar] [CrossRef] [PubMed]
- Jee, M.H.; Mraz, V.; Geisler, C.; Bonefeld, C.M. γδ T cells and inflammatory skin diseases. Immunol. Rev. 2020, 298, 61–73. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Eng. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Photobiology of vitamin D. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: London, UK, 2018; pp. 45–55. [Google Scholar]
- Graham, H.K.; Eckersley, A.; Ozols, M.; Mellody, K.T.; Sherratt, M.J. Human Skin: Composition, structure and visualisation methods. In Skin Biophysics, 1st ed.; Limbert, G., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–18. [Google Scholar]
- Lundborg, M.; Narangifard, A.; Wennberg, C.L.; Lindahl, E.; Daneholt, B.; Norlén, L. Human skin barrier structure and function analyzed by cryo-EM and molecular dynamics simulation. J. Struct. Biol. 2018, 203, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-based nanosystems as a tool to overcome skin barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef]
- Woodby, B.; Penta, K.; Pecorelli, A.; Lila, M.A.; Valacchi, G. Skin health from the inside out. Annu. Rev. Food Sci. Technol. 2020, 11, 235–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lademann, J.; Schanzer, S.; Richter, H.; Meinke, M.C.; Weigmann, H.J.; Patzelt, A. Stripping procedures for penetration measurements of topically applied substances. In Percutaneous Penetration Enhancers Drug Penetration into/through the Skin: Methodology and General Considerations, 1st ed.; Dragicevic, N., Maibach, H., Eds.; Springer: Berlin, Germany, 2017; pp. 205–214. [Google Scholar]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef]
- Wohlrab, J. Topical preparations and their use in dermatology. J. Dtsch. Dermatol. Ges. 2016, 14, 1061–1069. [Google Scholar] [CrossRef]
- Ribeiro, C.S.; Leal, F.; Jeunon, T. Skin anatomy, histology, and physiology. In Daily Routine in Cosmetic Dermatology. Clinical Approaches and Procedures in Cosmetic Dermatology; Issa, M., Tamura, B., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–12. [Google Scholar]
- Lavers, I. Exploring skin anatomy, function and site-specific treatment options. J. Aesthet. Nurs. 2017, 6, 172–180. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Chapter 3—Skin penetration of nanoparticles. In Micro and Nano Technologies, Emerging Nanotechnologies in Immunology; Shegokar, R., Souto, E.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–88. [Google Scholar]
- Montagna, W.; Ebling, F.J.G. Human Skin. Encyclopedia Britannica. 2021. Available online: https://www.britannica.com/science/human-skin (accessed on 19 October 2021).
- Osseiran, S.; Dela Cruz, J.; Jeong, S.; Wang, H.; Fthenakis, C.; Evans, C. Characterizing stratum corneum structure, barrier function, and chemical content of human skin with coherent Raman scattering imaging. Biomed. Opt. Express. 2018, 9, 6425–6443. [Google Scholar] [CrossRef] [PubMed]
- Yokose, U.; Ishikawa, J.; Morokuma, Y.; Naoe, A.; Inoue, Y.; Yasuda, Y.; Tsujimura, H.; Fujimura, T.; Murase, T.; Hatamochi, A. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Desmet, E.; van Gele, M.; Lambert, J. Topically applied lipid- and surfactant-based nanoparticles in the treatment of skin disorders. Expert Opin. Drug Del. 2017, 14, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Olmsted, P.D. The physics of stratum corneum lipid membranes. Phil. Trans. R. Soc. A 2016, 374, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2017, 29, 243–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezati, N.; Roberts, M.S.; Zhang, Q.; Moghimi, H.R. Measurement of Hansen Solubility Parameters of Human Stratum Corneum. Iran. J. Pharm. Res. 2020, 19, 572–578. [Google Scholar] [PubMed]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration in Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Walicka, A.; Iwanowska-Chomiak, B. Drug Diffusion Transport Through Human Skin. Int. J. Appl. Mech. Eng. 2018, 23, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, K.C.B.; Valle, A.B.C.d.S.; Paes, C.Q.; Tavares, G.D.; Pittella, F. Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances. Pharmaceutics 2021, 13, 1454. [Google Scholar] [CrossRef]
- Wolff, K.; Johnson, R.A.; Saavedra, A.P.; Roh, E.K. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2017; pp. 693–759. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2043§ionid=154893575 (accessed on 19 October 2021).
- Urban, K.; Chu, S.; Scheufele, C.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G.R. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int. 2020, 2, 22–27. [Google Scholar] [CrossRef]
- Craddock, L.N.; Schieke, S.M. Superficial fungal infection. In Fitzpatrick’s Dermatology, 9th ed.; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw-Hill: New York, NY, USA, 2019; Chapter 160; pp. 1–48. Available online: https://accessmedicine.mhmedical.com/content.aspx?sectionid=210432218&bookid=2570&Resultclick=2 (accessed on 19 October 2021).
- Silverberg, B. A Structured Approach to Skin and Soft Tissue Infections (SSTIs) in an Ambulatory Setting. Clin. Pract. 2021, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.R.; Margolis, D.J. Cellulitis and erysipelas. In Fitzpatrick’s Dermatology, 9th ed.; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw-Hill: New York, NY, USA, 2019; Chapter 151; pp. 1–19. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2570§ionid=210413306 (accessed on 19 October 2021).
- Sartelli, M.; Guirao, X.; Hardcastle, T.; Kluger, Y.; Boermeester, M.A.; Rasa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft tissue infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Falcone, M.; Concia, E.; Giusti, M.; Mazzone, A.; Santini, C.; Stefani, S.; Violi, F. Acute bacterial skin and skin structure infections in internal medicine wards: Old and new drugs. Inter. Emerg. Med. 2016, 11, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Khadr, L.; Harfouche, M.; Omori, R.; Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J. The Epidemiology of Herpes Simplex Virus Type 1 in Asia: Systematic Review, Meta-analyses, and Meta-regressions. Clin. Infect. Dis. 2019, 68, 757–772. [Google Scholar] [CrossRef]
- Poole, C.L.; Kimberlin, D.W. Antiviral Approaches for the Treatment of Herpes Simplex Virus Infections in Newborn Infants. Annu. Rev. Virol. 2018, 5, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Illzam, E.M.; Muniandy, R.K.; Sharifah, A.M.; Nang, M.K.; Ramesh, B. Herpes simplex virus infections, Pathophysiology and Management. IOSR J. Dent. Med. Sci. 2016, 15, 85–91. [Google Scholar] [CrossRef]
- Schwingen, J.; Kaplan, M.; Kurschus, F.C. Review-Current Concepts in Inflammatory Skin Diseases Evolved by Transcriptome Analysis: In-Depth Analysis of Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2020, 21, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.W.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Golan, Y. Current Treatment Options for Acute Skin and Skin-structure Infections. Clin. Infect. Dis. 2019, 68, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktas, E.; Esin, M.N. Skin disease symptoms and related risk factors among young workers in high-risk jobs. Contact Dermat. 2016, 75, 96–105. [Google Scholar] [CrossRef]
- Münch, S.; Wohlrab, J.; Neubert, R.H.H. Dermal and transdermal delivery of pharmaceutically relevant macromolecules. Eur. J. Pharm. Biopharm. 2017, 119, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Transdermal Drug Delivery-Technologies, Markets and Companies; Jain PharmaBiotech: Basel, Switzerland, 2021; pp. 1–302. [Google Scholar]
- Alany, R. Topical and Transdermal Formulation and Drug Delivery. Pharm. Dev. Technol. 2017, 22, 457. [Google Scholar] [CrossRef]
- Brunaugh, A.D.; Smyth, H.D.C.; Williams, R.O., III. Topical and transdermal drug delivery. In Essential Pharmaceutics; AAPS Introductions in the Pharmaceutical Sciences; Springer: Cham, Switzerland, 2019; pp. 131–147. [Google Scholar]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postep. Derm. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, A. Retinoids in Acne Management: Review of Current Understanding, Future Considerations, and Focus on Topical Treatments. J. Drugs Dermatol. 2018, 17, 51–55. [Google Scholar]
- Temova Rakuša, Ž.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive Stability Study of Vitamin D3 in Aqueous Solutions and Liquid Commercial Products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Kawashima, M.; Ito, M.; Tsubota, K. Clinical safety and efficacy of vitamin D3 analog ointment for treatment of obstructive meibomian gland dysfunction. BMC Ophthalmol. 2017, 17, 84. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, H.; Deshmukh, G.; Dhoot, D.; Mamadi, R.; Barkate, H. Comparative Clinical Assessment of Effectiveness and Safety of Calcitriol and Calcipotriol in Mild Plaque Psoriasis. Int. J. Clin. Dermatol. 2020, 3, 28–31. [Google Scholar]
- Singh, R.K.; Lee, K.M.; Jose, M.V.; Nakamura, M.; Ucmak, D.; Farahnik, B.; Abrouk, M.; Zhu, T.H.; Bhutani, T.; Liao, W. The Patient’s Guide to Psoriasis Treatment. Part 1: UVB Phototherapy. Dermatol. Ther. 2016, 6, 307–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Rovati, C.; Arisi, M.; Tomasi, C.; Calzavara-Pinton, I.; Venturini, M.; Calzavara-Pinton, P. A Short Cycle of Narrow-Band UVB Phototherapy in the Early Phase of Dupilumab Therapy Can Provide a Quicker Improvement of Severe Atopic Dermatitis. Dermatology 2021, 237, 407–415. [Google Scholar] [CrossRef]
- Lossius, A.H.; Sundnes, O.; Ingham, A.C.; Edslev, S.M.; Bjørnholt, J.V.; Lilje, B.; Bradley, M.; Asad, S.; Haraldsen, G.; Skytt-Andersen, P.; et al. Shifts in the Skin Microbiota after UVB Treatment in Adult Atopic Dermatitis. Dermatology 2021, 1–12. [Google Scholar] [CrossRef]
- Eicher, L.; Knop, M.; Aszodi, N.; Senner, S.; French, L.; Wollenberg, A. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease-strategies for optimizing treatment outcome. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2253–2263. [Google Scholar] [CrossRef]
- Leppert, W.; Malec–Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.; Singh, S.; Gupta, S. Topical antimicrobial therapy: Current status and challenges. Indian J. Med. Microbiol. 2019, 37, 299–308. [Google Scholar] [CrossRef]
- Wohlrab, J. Neue Entwicklungen bei Topika. Hautarzt 2019, 70, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sharma, G.S.; Goyal, A.K.; Ghosh, G.; Si, S.C.; Rath, G. Recent advances in topical carriers of anti-fungal agents. Heliyon 2020, 6, e04663. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M. Skin penetration/permeation success determinants of nanocarriers: Pursuit of a perfect formulation. Colloids Surf. B. 2021, 203, 111748. [Google Scholar] [CrossRef] [PubMed]
- Chella, N.; Shastri, N.R. Lipid Carriers: Role and Applications in Nano Drug Delivery. In Particulate Technology for Delivery of Therapeutics; Jana, S., Jana, S., Eds.; Springer: Singapore, 2017; pp. 253–289. [Google Scholar]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Khan, M.M.; Parveen, F.; Torchilin, V. Lipid-Based Drug Delivery Systems in Regenerative Medicine. Materials 2021, 14, 5371. [Google Scholar] [CrossRef] [PubMed]
- LePree, J.M. Lipid based delivery—Are lipid-based drug delivery systems in your formulation toolbox? Drug Dev. Deliv. 2017, 17, 20–25. Available online: https://drug-dev.com/lipid-based-delivery-are-lipid-based-drug-delivery-systems-in-your-formulation-toolbox/ (accessed on 19 October 2021).
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug. Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Chaurasiya, P.; Ganju, E.; Upmanyu, N.; Ray, S.K.; Jain, P. Transfersomes: A novel technique for transdermal drug delivery. J. Drug Deliv. Ther. 2019, 9, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Nainwal, N.; Jawla, S.; Singh, R.; Saharan, V.A. Transdermal applications of ethosomes—A detailed review. J. Liposome Res. 2019, 29, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Liu, M.; Wen, J.; Sharma, M. Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status. Curr. Pharm. Des. 2020, 26, 3203–3217. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhao, Y.; Wang, L.; Xu, L.; Chen, X.; Han, J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. AAPS Pharm. Sci. Tech. 2020, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.H.S.; Delello Di Filippo, L.; Duarte, J.L.; Spósito, L.; de Camargo, B.A.F.; da Silva, P.B.; Chorilli, M. Exploiting solid lipid nanoparticles and nanostructured lipid carriers for drug delivery against cutaneous fungal infections. Crit. Rev. Microbiol. 2020, 47, 79–90. [Google Scholar] [CrossRef]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomedicine 2017, 13, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Sguizzato, M.; Bories, C.; Nastruzzi, C.; Cortesi, R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers 2018, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Waghule, T.; Gorantla, S.; Rapalli, V.K.; Shah, P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Emerging Trends in Topical Delivery of Curcumin Through Lipid Nanocarriers: Effectiveness in Skin Disorders. AAPS Pharm. Sci. Tech. 2020, 21, 284. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Mezei, M.; Gulasekharam, V. Liposomes—A selective drug delivery system for the topical route of administration I. Lotion dosage form. Life Sci. 1980, 26, 1473–1477. [Google Scholar] [CrossRef]
- Lucia, M. Lipid-Based Nanoparticles as Carriers for Dermal Delivery of Antioxidants. Curr. Drug. Metab. 2017, 18, 469–480. [Google Scholar] [PubMed]
- Yang, M.; Gu, Y.; Tang, X.; Wang, T.; Liu, J. Advancement of Lipid-Based Nanocarriers and Combination Application with Physical Penetration Technique. Curr. Drug. Deliv. 2019, 16, 312–324. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.; Lobão, P. Non-Steroidal Anti-Inflammatory Drugs Loaded Liposomes for Topical Treatment of Inflammatory and Degenerative Conditions. Curr. Med. Chem. 2020, 27, 3809–3829. [Google Scholar] [CrossRef] [PubMed]
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef]
- Lai, F.; Caddeo, C.; Manca, M.L.; Manconi, M.; Sinico, C.; Fadda, A.M. What’s new in the field of phospholipid vesicular nanocarriers for skin drug delivery. Int. J. Pharm. 2020, 583, 119398. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Formulation and optimisation of novel transfersomes for sustained release of local anaesthetic. J. Pharm. Pharmacol. 2019, 71, 1508–1519. [Google Scholar] [CrossRef]
- Touitou, E. Compositions for Applying Active Substances to or through the Skin. US Patent 5,716,638, 10 February 1998. [Google Scholar]
- Ita, K. Current Status of Ethosomes and Elastic Liposomes in Dermal and Transdermal Drug Delivery. Curr. Pharm. Des. 2016, 22, 5120–5126. [Google Scholar] [CrossRef]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020, 15, 119278. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Nanda, S. Quality by design driven development of resveratrol loaded ethosomal hydrogel for improved dermatological benefits via enhanced skin permeation and retention. Int. J. Pharm. 2019, 567, 118448. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- Lalotra, A.S.; Singh, V.; Khurana, B.; Agrawal, S.; Shrestha, S.; Arora, D. A Comprehensive Review on Nanotechnology-Based Innovations in Topical Drug Delivery for the Treatment of Skin Cancer. Curr. Pharm. Des. 2020, 26, 5720–5731. [Google Scholar] [CrossRef]
- Pandey, K. An Overview on Promising Nanotechnological Approaches for the Treatment of Psoriasis. Recent Pat. Nanotechnol. 2020, 14, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Ali, J.; Baboota, S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018, 57, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Vaidya, A.; Sharma, V. Confronting Penetration Threshold via Fluidic Terpenoid Nanovesicles. Curr. Drug. Deliv. 2018, 15, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Verma, S.; Singh, M.; Chalotra, T.; Utreja, P. Advanced Drug Delivery Systems for Transdermal Delivery of Non-Steroidal Anti-Inflammatory Drugs: A Review. Curr. Drug. Deliv. 2018, 15, 1087–1099. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Shen, L.N.; Wu, Z.H.; Zhao, J.H.; Feng, N.P. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int. J. Pharm. 2014, 471, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Nagra, U.; Zaman, M.; Mahmood, A.; Barkat, K. Lipid Vesicles and Nanoparticles for Non-invasive Topical and Transdermal Drug Delivery. Curr. Pharm. Des. 2020, 26, 2149–2166. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.C.; Xu, X.L.; Lou, X.F.; Du, Y.Z. Recent Progress and Future Directions: The Nano-Drug Delivery System for the Treatment of Vitiligo. Int. J. Nanomed. 2020, 15, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Iranpanah, A.; Najafi, F.; Belwal, T.; Ramola, S.; Abbasabadi, Z.; Momtaz, S.; Farzaei, M.H. Implications of grape extract and its nanoformulated bioactive agent resveratrol against skin disorders. Arch. Dermatol. Res. 2019, 311, 577–588. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Baveloni, F.G.; Riccio, B.V.F.; Di Filippo, L.D.; Fernandes, M.A.; Meneguin, A.B.; Chorilli, M. Nanotechnology-based Drug Delivery Systems as Potential for Skin Application: A Review. Curr. Med. Chem. 2021, 28, 3216–3248. [Google Scholar] [CrossRef]
- Kurangi, B.; Jalalpure, S.; Jagwani, S. Formulation and Evaluation of Resveratrol Loaded Cubosomal Nanoformulation for Topical Delivery. Curr. Drug. Deliv. 2021, 18, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Badie, H.; Abbas, H. Novel small self-assembled resveratrol-bearing cubosomes and hexosomes: Preparation, charachterization, and ex vivo permeation. Drug Dev. Ind. Pharm. 2018, 44, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.; Ahmad, Z.; Khan, A.A.; Akhtar, J.; Singh, S.P.; Ahmad, F.J. Strategies in Development and Delivery of Nanotechnology Based Cosmetic Products. Drug Res. 2018, 68, 545–552. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, J.F.; Routh, A.F. Coated colloidosomes as novel drug delivery carriers. Exp. Opin. Drug Del. 2019, 16, 903–906. [Google Scholar] [CrossRef] [Green Version]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Mahtab, A.; Siddiqui, L.; Singh, A.; Gautam, N.; Rabbani, S.A.; Goel, H.; Talegaonkar, S. Biocompatible Nanovesicular Drug Delivery Systems with Targeting Potential for Autoimmune Diseases. Curr. Pharm. Des. 2020, 26, 5488–5502. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.; Sherje, A.P. Nano intervention in topical delivery of corticosteroid for psoriasis and atopic dermatitis-a systematic review. J. Mat. Sci. Mater. Med. 2021, 32, 88. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Fangueiro, J.F.; Faggio, C.; Santini, A.; Souto, E.B. Archaeosomes for Skin Injuries. In Carrier-Mediated Dermal Delivery: Applications in the Prevention and Treatment of Skin Disorders; Ascenso, A., Ribeiro, H., Simões, S., Eds.; Pan Stanford Publishing: Singapore, 2017; pp. 323–355. [Google Scholar]
- Sallam, M.A.; Prakash, S.; Kumbhojkar, N.; Shields, C.W.; Mitragotri, S. Formulation-based approaches for dermal delivery of vaccines and therapeutic nucleic acids: Recent advances and future perspectives. Bioeng. Transl. Med. 2021, 6, e10215. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chen, C.H.; Wu, H.Y.; Tsai, N.M.; Jian, T.Y.; Chang, Y.C.; Lin, C.H.; Wu, C.H.; Hsu, F.T.; Leung, T.K.; et al. Inhibition of breast cancer with transdermal tamoxifen-encapsulated lipoplex. J. Nanobiotechnol. 2016, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Kushwaha, P.; Ahmad, U.; Abdullah, M. Proliposomes: An Approach for the Development of Stable Liposome. Ars. Pharm. 2019, 60, 231–240. [Google Scholar]
- Hiremath, N.; Gowda, D.V.; Anusha, R.; Shamant, B.S.; Srivastava, A.; Moin, A. Proliposomes: A novel approach to carrier drug delivery system. J. Chem. Pharm. Res. 2016, 8, 348–354. [Google Scholar]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Kotla, N.G.; Chandrasekar, B.; Rooney, P.; Sivaraman, G.; Larrañaga, A.; Krishna, K.V.; Pandit, A.; Rochev, Y. Biomimetic Lipid- Based Nanosystems for Enhanced Dermal Delivery of Drugs and Bioactive Agents. ACS Biomater. Sci. Eng. 2017, 3, 1262–1272. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef]
- Sureka, S.; Gupta, G.; Agarwal, M.; Mishra, A.; Singh, S.K.; Singh, R.P.; Sah, S.K.; de Jesus, A.; Pinto, T.; Dua, K. Formulation, In-Vitro and Ex-Vivo Evaluation of Tretinoin Loaded Cubosomal Gel for the Treatment of Acne. Recent Pat. Drug Deliv. Formul. 2018, 12, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Magana, J.R.; Esquena, J.; Solans, C.; Rodriguez-Abreu, C. Deconstruction of technical grade diglycerol isostearate enables the controlled preparation of hexosomes and liposomes. J. Molec. Liq. 2021, 343, 117594. [Google Scholar] [CrossRef]
- Fornasier, M.; Pireddu, R.; Del Giudice, A.; Sinico, C.; Nylander, T.; Schillén, K.; Galantini, L.; Murgia, S. Tuning lipid structure by bile salts: Hexosomes for topical administration of catechin. Colloids Surf. B. Biointerfaces 2021, 199, 111564. [Google Scholar] [CrossRef] [PubMed]
- Gururaj, S.S.; Ashok, V.B.; Swati, S.M.; Nilesh, R.B.; Prashant, H.K.; Nishant, P.K.; Sagar, T. An overview on nanocarrier technology Aquasomes. J. Pharm. Res. 2009, 2, 1174–1177. [Google Scholar]
- Lopez, C.; Mériadec, C.; David-Briand, E.; Dupont, A.; Bizien, T.; Artzner, F.; Riaublanc, A.; Anton, M. Loading of lutein in egg-sphingomyelin vesicles as lipid carriers: Thermotropic phase behaviour, structure of sphingosome membranes and lutein crystals. Food Res Int. 2020, 138, 109770. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Fang, Y.; Ma, L. The self-crosslinked ufasome of conjugated linoleic acid: Investigation of morphology, bilayer membrane and stability. Colloids Surf. B. Biointerfaces 2014, 123, 8–14. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; AbouGhaly, M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box–Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef]
- Perez, A.P.; Altube, M.J.; Schilrreff, P.; Apezteguia, G.; Celes, F.S.; Zacchino, S.; de Oliveira, C.I.; Romero, E.L.; Morilla, M.J. Topical amphotericin B inultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces 2016, 139, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.J.; Garg, N.K.; Beg, S.; Singh, B.; Katare, O.P. Nanosized ethosomes-based hydrogel formulations of methoxsalen for en- hanced topical delivery against vitiligo: Formulation optimization, in vitro evaluation and preclinical assessment. J. Drug Target. 2016, 24, 233–246. [Google Scholar] [CrossRef]
- Oge, L.K.; Broussard, A.; Marshall, M.D. Acne Vulgaris: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 475–484. [Google Scholar]
- Sevimli Dikicier, B. Topical treatment of acne vulgaris: Efficiency, side effects, and adherence rate. J. Int. Med. Res. 2019, 47, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Apriani, E.F.; Rosana, Y.; Iskandarsyah, I. Formulation, characterization, and in vitro testing of azelaic acid ethosome-based cream against Propionibacterium acnes for the treatment of acne. J. Adv. Pharm. Technol. Res. 2019, 10, 75. [Google Scholar]
- Schaeffer, H.J.; Beauchamp, L.; de Miranda, P.; Elion, G.B.; Bauer, D.J.; Collins, P. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 1978, 272, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.; Pisanty, S.; Czerninski, R.; Helser, M.; Eliav, E.; Touitou, E. A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 87, 700–705. [Google Scholar] [CrossRef]
- Shukla, K.V.; Sharma, A.; Yadav, M. Formulation development and evaluation of ethosomal gel of acyclovir for the treatment of herpes zoster. J. Nanosci. Nanotechnol. 2019, 9 (Suppl. 2), 664–668. [Google Scholar]
- Babaie, S.; Ghanbarzadeh, S.; Davaran, S.; Kouhsoltani, M.; Hamishehkar, H. Nanoethosomes for Dermal Delivery of Lidocaine. Adv. Pharm. Bull. 2015, 5, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godin, B.; Touitou, E.; Rubinstein, E.; Athamna, A.; Athamna, M. A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: An in vivo study. J. Antimicrob. Chemother. 2005, 55, 989–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godin, B.; Touitou, E. Erythromycin ethosomal systems: Physicochemical characterization and enhanced antibacterial activity. Curr. Drug Deliv. 2005, 2, 269–275. [Google Scholar] [CrossRef]
- Zahid, S.R.; Dangi, S.; Shende, S.M.; Upmanyu, N. Formulation and evaluation of clindamycin phosphate ethosomal gel. World J. Pharm. Pharm. Sci. 2020, 9, 1804–1813. [Google Scholar]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Di Marzio, L.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Diez-Sales, O.; Ruiz-Sauri, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernandez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; El Ramly, A. Tretinoin-loaded liposomal formulations: From lab to comparative clinical study in acne patients. Drug Deliv. 2016, 23, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, S.G.; Škalko-Basnet, N.; Jacobsen, C.d.A.C.; Holsæter, A.M. Successful co-encapsulation of benzoyl peroxide and chloramphenicol in liposomes by a novel manufacturing method-dual asymmetric centrifugation. Eur. J. Pharm. Sci. 2017, 97, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Kale, D.P.; Swami, R.; Katiyar, S.S. Codelivery of benzoyl peroxide & adapalene using modified liposomal gel for improved acne therapy. Nanomedicine 2018, 13, 1481–1493. [Google Scholar] [PubMed]
- Sarwa, K.K.; Mazumder, B.; Rudrapal, M.; Verma, V.K. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2015, 22, 638–646. [Google Scholar] [CrossRef]
- Khan, M.A.; Pandit, J.; Sultana, Y.; Sultana, S.; Ali, A.; Aqil, M.; Chauhan, M. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: In vitro characterization and in vivo study. Drug Deliv. 2015, 22, 795–802. [Google Scholar] [CrossRef]
- Dorrani, M.; Garbuzenko, O.B.; Minko, T.; Michniak-Kohn, B. Development of edge-activated liposomes for siRNA delivery to human basal epidermis for melanoma therapy. J. Control Release 2016, 228, 150–158. [Google Scholar] [CrossRef]
- Desmet, E.; Bracke, S.; Forier, K.; Taevernier, L.; Stuart, M.C.A.; De Spiegeleer, B.; Raemdonck, K.; Van Gele, M.; Lambert, J. An elastic liposomal formulation for RNAi-based topical treatment of skin disorders: Proof-of-concept in the treatment of psoriasis. Int. J. Pharm. 2016, 500, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Samy, N.; Nasr, M.; Alyoussef, A.A. Topical colloidal indocyanine green-mediated photodynamic therapy for treatment of basal cell carcinoma. Pharm. Dev. Technol. 2017, 22, 545–550. [Google Scholar] [CrossRef]
- Gupta, M.; Prajapati, R.N.; Irchhaiya, R.; Singh, N.; Prajapati, S.K. Novel clindamycin loaded transfersomes formulation for effective management of acne. World J. Pharm. Res. 2017, 6, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Wang, T.; Li, T.; Ma, Y.; Shen, S.; He, B.; Mo, R. Enhanced Transdermal Drug Delivery by Transfer-some-Embedded Oligopeptide Hydrogel for Topical Chemotherapy of Melanoma. ACS Nano 2018, 12, 9693–9701. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.J.; Din, F.U.; Khan, G.M. Sodium stibogluconate loaded nano-deformable liposomes for topical treatment of leishmaniasis: Macrophage as a target cell. Drug Deliv. 2018, 25, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.M.; Hasan, O.A.; El Sisi, A.M. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: A promising approach for enhancement of skin permeation. Int. J. Nanomed. 2019, 14, 1551–1562. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Dar, M.J.; McElroy, C.A.; Khan, M.I.; Satoskar, A.R.; Khan, G.M. Development and evaluation of novel miltefosine-polyphenol co-loaded second generation nano-transfersomes for the topical treatment of cutaneous leishmaniasis. Expert Opin. Drug Deliv. 2020, 17, 97–110. [Google Scholar] [CrossRef]
- Harmita, H.; Iskandarsyah, I.; Afifah, S.F. Effect of transfersome formulation on the stability and antioxidant activity of N- acetylcysteine in anti-aging cream. Int. J. Appl. Pharm. 2020, 12, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Marto, J.; Vitor, C.; Guerreiro, A.; Severino, C.; Eleuterio, C.; Ascenso, A.; Simoes, S. Ethosomes for enhanced skin delivery of griseofulvin. Colloids Surf. B Biointerfaces 2016, 146, 616–623. [Google Scholar] [CrossRef]
- Yu, Z.; Lv, H.; Han, G.; Ma, K. Ethosomes loaded with cryptotanshinone for acne treatment through topical gel formulation. PLoS ONE 2016, 11, e0159967. [Google Scholar] [CrossRef] [Green Version]
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 2019, 137, 104972. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, H.M. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187. [Google Scholar] [CrossRef]
- Richa, P.; Kumar, S.D.; Kumar, S.A. Formulation and evaluation of ethosomes of clobetasol propionate. World J. Pharm. Res. 2016, 5, 1183–1197. [Google Scholar]
- Mishra, R.; Shende, S.; Jain, P.K.; Jain, V. Formulation and evaluation of gel containing ethosomes entrapped with tretinoin. J. Drug Deliv. Ther. 2018, 8, 315–321. [Google Scholar] [CrossRef]
- Pando, D.; Matos, M.; Gutierrez, G.; Pazos, C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf. B Biointerfaces 2015, 128, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Moghddam, S.R.M.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater. Sci. Eng. C 2016, 69, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Sun, L.; Wang, L.; Lin, Z.; Liu, Z.; Xi, L.; Wang, Z.; Zheng, Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf. B Biointerfaces 2019, 182, 110352. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and in Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Biol. Mater. 2020, 3, 4198–4207. [Google Scholar] [CrossRef]
- Khan, S.; Jain, P.; Jain, S.; Jain, R.; Bhargava, S.; Jain, A. Topical Delivery of Erythromycin through Cubosomes for Acne. Pharm. Nanotechnol. 2018, 6, 38–47. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Banerjee, S.; Khan, S.; Jha, P.N.; Gupta, G.; Dua, K.; Hasnain, M.S.; Nayak, A.K.; Dubey, S.K.; Singhvi, G. QbD-driven formulation development and evaluation of topical hydrogel containing ketoconazole loaded cubosomes. Mater. Sci. Eng. C 2021, 119, 111548. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.K.; Handa, V.; Kathuria, H. Oleic Acid Nanovesicles of Minoxidil for Enhanced Follicular Delivery. Medicines 2018, 5, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.C.; Amaral, M.H.; Lobo, J.M.; Lopes, C.M. Lipid nanoparticles for the delivery of biopharmaceuticals. Curr. Pharm. Biotechnol. 2015, 16, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, H.; Wu, B.; Zhang, X. Lipid nanoparticles for the delivery of active natural medicines. Curr. Pharm. Des. 2017, 23, 6705–6713. [Google Scholar] [CrossRef]
- De Souza, M.L.; Dos Santos, W.M.; de Sousa, A.L.M.D.; de Albuquerque Wanderley Sales, V.; Nóbrega, F.P.; de Oliveira, M.V.G.; Rolim-Neto, P.J. Lipid Nanoparticles as a Skin Wound Healing Drug Delivery System: Discoveries and Advances. Curr. Pharm. Des. 2020, 26, 4536–4550. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Gratieri, T.; Krawczyk-Santos, A.P.; da Rocha, P.B.; Gelfuso, G.M.; Marreto, R.N.; Taveira, S.F. SLN- and NLC-Encapsulating Antifungal Agents: Skin Drug Delivery and their Unexplored Potential for Treating Onychomycosis. Curr. Pharm. Des. 2017, 23, 6684–6695. [Google Scholar] [CrossRef]
- Pham, D.T.T.; Tran, P.H.L.; Tran, T.T.D. Development of solid dispersion lipid nanoparticles for improving skin delivery. Saudi Pharm. J. 2019, 27, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Rapalli, V.K.; Gorantla, S.; Saha, R.N.; Dubey, S.K.; Puri, A.; Singhvi, G. Nanostructured Lipid Carriers as Potential Drug Delivery Systems for Skin Disorders. Curr. Pharm. Des. 2020, 26, 4569–4579. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, K. Solid Lipid Nanoparticles: A Promising Nanomaterial in Drug Delivery. Curr. Pharm. Des. 2019, 25, 3943–3959. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Trivino, A.; Gumireddy, A.; Chauhan, H. Drug-Lipid-Surfactant Miscibility for the Development of Solid Lipid Nanoparticles. AAPS Pharm. Sci. Tech. 2019, 20, 46. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Sahafi, S.M. Formulation and characterization of novel nanostructured lipid carriers made from beeswax, propolis wax and pomegranate seed oil. Food Chem. 2018, 244, 83–92. [Google Scholar] [CrossRef]
- Sebaaly, C.; Charcosset, C.; Stainmesse, S.; Fessi, H.; Greige-Gerges, H. Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous and lyophilized states: From laboratory to large scale using a membrane contactor. Carbohydr. Polym. 2016, 138, 75–85. [Google Scholar] [CrossRef]
- Shah, M.K.; Khatri, P.; Vora, N.; Patel, N.K.; Jain, S.; Lin, S. Lipid nanocarriers: Preparation, characterization and absorption mechanism and applications to improve oral bioavailability of poorly water-soluble drugs. In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 117–147. [Google Scholar]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.A.; Patel, D.M.; Patel, J.K.; Patel, D.H. Solvent Emulsification Evaporation and Solvent Emulsification Diffusion Techniques for Nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer: Cham, Switzerland, 2021; pp. 287–300. [Google Scholar]
- Singh, D.; Tiwary, A.K.; Bedi, N. Self-microemulsifying Drug Delivery System for Problematic Molecules: An Update. Recent Pat. Nanotech. 2019, 13, 92–113. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, L.B.; Petersson, K.; Nielsen, H.M. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J. Pharm. Biopharm. 2011, 79, 68–75. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formula-tions for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl. Res. 2017, 7, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Messina, C.M.; Manuguerra, S.; Santagati, L.M.; Pasquinucci, L.; Turnaturi, R.; Parenti, C.; Arena, R.; Santulli, A. In Vitro Antioxidant Activity and In Vivo Topical Efficacy of Lipid Nanoparticles Co-Loading Idebenone and Tocopheryl Acetate. Appl. Sci. 2019, 9, 845. [Google Scholar] [CrossRef] [Green Version]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Gad, H.A.; El-Rahman, F.A.A.; Hamdy, G.M. Chamomile oil loaded solid lipid nanoparticles: A naturally formulated remedy to enhance the wound healing. J. Drug Deliv. Sci. Technol. 2019, 50, 329–338. [Google Scholar] [CrossRef]
- Butani, D.; Yewale, C.; Misra, A. Topical Amphotericin B solid lipid nanoparticles: Design and development. Colloids Surf. B Biointerfaces 2016, 139, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; do Céu Teixeira, M.; do Céu Sousa, M.; Martins-Gomes, C.; Silva, A.M.; Souto, E.M.B.; Musumeci, T. Clotrima- zole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics 2019, 11, 231. [Google Scholar] [CrossRef] [Green Version]
- Ghate, V.M.; Lewis, S.A.; Prabhu, P.; Dubey, A.; Patel, N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016, 108, 253–261. [Google Scholar] [CrossRef]
- Malik, D.S.; Kaur, G. Exploring therapeutic potential of azelaic acid loaded NLCs for the treatment of acne vulgaris. J. Drug Deliv. Sci. Technol. 2020, 55, 101418. [Google Scholar] [CrossRef]
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016, 33, 372–380. [Google Scholar] [CrossRef]
- Harde, H.; Agrawal, A.K.; Katariya, M.; Kale, D.; Jain, S. Development of a topical adapalene-solid lipid nanoparticle load-ed gel with enhanced efficacy and improved skin tolerability. RSC Adv. 2015, 5, 43917–43929. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Influence of selected variables on fabrication of Triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif. Cells Nanomed. Biotechnol. 2016, 44, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Car-riers. AAPS Pharm. Sci. Tech. 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Shrotriya, S.N.; Vidhate, B.V.; Shukla, M.S. Formulation and development of Silybin loaded solid lipid nanoparticle enriched gel for irritant contact dermatitis. J. Drug Deliv. Sci. Technol. 2017, 41, 164–173. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Flucona-zole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef]
- Aland, R.; Ganesan, M.; Rajeswara, R.P. Development and optimization of tazarotene loaded solid lipid nanoparticles for topical delivery. Asian J. Pharm. Clin. Res. 2019, 12, 63–77. [Google Scholar] [CrossRef]
- Al-Maghrabi, P.M.; Khafagy, E.-S.; Ghorab, M.M.; Gad, S. Influence of formulation variables on miconazole nitrate-loaded lipid based nanocarrier for topical delivery. Colloids Surf. B Biointerfaces 2020, 193, 111046. [Google Scholar] [CrossRef]
- Bhalekar, M.; Upadhaya, P.; Madgulkar, A. Formulation and evaluation of Adapalene-loaded nanoparticulates for epidermal localization. Drug Deliv. Transl. Res. 2015, 5, 585–595. [Google Scholar] [CrossRef]
- Gupta, S.; Wairkar, S.; Bhatt, L.K. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J. Microencapsul. 2020, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kelidari, H.R.; Saeedi, M.; Hajheydari, Z.; Akbari, J.; Morteza-Semnani, K.; Akhtari, J.; Valizadeh, H.; Asare-Addo, K.; Nokhodchi, A. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate ac-ne vulgaris: A randomized, double-blind, prospective trial. Colloids Surf. B Biointerfaces 2016, 146, 47–53. [Google Scholar] [CrossRef]
- Nagaich, U.; Gulati, N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: Design and in vivo characterization. Drug Deliv. Transl. Res. 2016, 6, 289–298. [Google Scholar] [CrossRef]
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Ciampo, J.O.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.S.; De Martino, L.C.; Dartora, V.F.C.; De Araujo, G.L.B.; Ishida, K.; Lopes, L.B. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur. J. Pharm. Sci. 2020, 149, 105296. [Google Scholar] [CrossRef]
- Madan, J.R.; Khobaragade, S.; Dua, K.; Awasthi, R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol. Ther. 2020, 33, e13370. [Google Scholar] [CrossRef]
- Sathe, P.; Saka, R.; Kommineni, N.; Raza, K.; Khan, W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev. Ind. Pharm. 2019, 45, 826–838. [Google Scholar] [CrossRef]
- Waghule, T.; Rapalli, V.K.; Singhvi, G.; Manchanda, P.; Hans, N.; Dubey, S.K.; Hasnain, M.S.; Nayak, A.K. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 303–315. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, K.; Bedi, N. Topical Nanostructured Lipid Carrier Based Hydrogel of Mometasone Furoate for the Treatment of Psoriasis. Pharm. Nanotechnol. 2018, 6, 133–143. [Google Scholar] [CrossRef]
- Lewies, A.; Wentzel, J.F.; Jordaan, A.; Bezuidenhout, C.; Du Plessis, L.H. Interactions of the antimicrobial peptide nisin Z with conventional antibiotics and the use of nanostructured lipid carriers to enhance antimicrobial activity. Int. J. Pharm. 2017, 526, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Garg, N.K.; Jain, A.; Kesharwani, P.; Jain, A.K.; Nirbhavane, P.; Tyagi, R.K. A synergistic approach of adapalene-loaded nanostructured lipid carriers, and vitamin C co-administration for treating acne. Drug Dev. Ind. Pharm. 2016, 42, 897–905. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2009, 99, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, K.H. Nanotechnology in medicine and relevance to dermatology: Present concepts. Indian J. Dermatol. 2012, 57, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.L. Nanotechnology: Challenging the limit of creativity in targeted drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1527–1528. [Google Scholar] [CrossRef]
- Heuschkel, S.; Goebel, A.; Neubert, R.H.H. Microemulsions-modern colloidal carrier for dermal and transdermal drug delivery. J. Pharm. Sci. 2007, 97, 603–631. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Solanki, A.B.; Patel, B.G. Investigating effect of microemulsion components: In vitro permeation of ketoconazole. Pharm. Dev. Technol. 2011, 16, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nanoemulsion templates—A review. J. Control Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Badnjevic, A. CMBEBIH 2017: Proceedings of the International Conference on Medical and Biological Engineering 2017; Springer: New York, NY, USA, 2017; p. 825. [Google Scholar]
- Kaur, A.; Katiyar, S.S.; Kushwah, V.; Jain, S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomedicine 2017, 13, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Rajitha, P.; Shammika, P.; Aiswarya, S.; Gopikrishnan, A.; Jayakumar, R.; Sabitha, M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2019, 49, 463–476. [Google Scholar] [CrossRef]
- Coneac, G.; Vlaia, V.; Olariu, I.; Mut, A.M.; Anghel, D.F.; Ilie, C.; Popoiu, C.; Lupuleasa, D.; Vlaia, L. Development and Evaluation of New Microemulsion-Based Hydrogel Formulations for Topical Delivery of Fluconazole. AAPS PharmSciTech 2015, 16, 889–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduno-Ramirez, M.L.; Clares, B.; Garcia, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Kaur, R.; Kaur, R. An ionic liquid-in-water microemulsion as a potential carrier for topical delivery of poorly water soluble drug: Development, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2015, 495, 913–923. [Google Scholar] [CrossRef]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 2017, 18, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Katare, O.P. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J. Microencapsul. 2015, 32, 419–431. [Google Scholar] [CrossRef]
- Pham, J.; Nayel, A.; Hoang, C.; Elbayoumi, T. Enhanced effectiveness of tocotrienol-based nano-emulsified system for topical delivery against skin carcinomas. Drug Deliv. 2016, 23, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.; Abdel-Hamid, S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev. Ind. Pharm. 2016, 42, 636–643. [Google Scholar] [CrossRef]
- Amarji, B.; Garg, N.K.; Singh, B.; Katare, O.P. Microemulsions mediated effective delivery of methotrexate hydrogel: More than a tour de force in psoriasis therapeutics. J. Drug Target. 2016, 24, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Abdel-Hamid, S.; Moftah, N.H.; Fadel, M.; Alyoussef, A.A. Jojoba Oil Soft Colloidal Nanocarrier of a Synthetic Retinoid: Preparation, Characterization and Clinical Efficacy in Psoriatic Patients. Curr. Drug Deliv. 2017, 14, 426–432. [Google Scholar] [CrossRef]
- Kumari, B.; Kesavan, K. Effect of chitosan coating on microemulsion for effective dermal clotrimazole delivery. Pharm. Dev. Technol. 2017, 22, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Salimi, A.; Changizi, S. Preparation and microstructural characterization of Griseofulvin microemulsions using different experimental methods: SAXS and DSC. Adv. Pharm. Bull. 2017, 7, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, A.M.; Ammar, N.M.; Hussein, R.A.; Mostafa, D.M.; Basha, M.; Abdel Hamid, M.F. Boswellia carterii Birdwood topical microemulsion for the treatment of inflammatory dermatological conditions; a prospective study. Trop. J. Nat. Prod. Res. 2020, 4, 372–377. [Google Scholar]
- Khiljee, S.; Ur Rehman, N.; Khiljee, T.; Loebenberg, R.; Ahmad, R.S. Formulation and clinical evaluation of topical dosage forms of Indian Penny Wort, walnut and turmeric in eczema. Pak. J. Pharm. Sci. 2015, 28, 2001–2007. [Google Scholar]
- Jagdale, S.; Chaudhari, B. Optimization of microemulsion based transdermal gel of triamcinolone. Recent Pat. Anti-Infect. Drug Discov. 2017, 12, 61–78. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for improved topical delivery of retinyl palmitate: Formulation design and stability evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of triptolide-nanoemulsion gels for percutaneous administration: Physicochemical, transport, pharmacokinetic and pharmacodynamic characteristics. J. Nano Biotechnol. 2017, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Lee, S.H.; Chia, V.D.; Chow, P.S.; MacBeath, C.; Liu, Y.; Shlieout, G. Development of microemulsion based topical ivermectin formulations: Pre-formulation and formulation studies. Colloids Surf. B Biointerfaces 2020, 189, 110823. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Maulvi, F.A.; Patel, P.S.; Shukla, M.R.; Shah, K.M.; Gupta, A.R.; Joshi, S.V.; Shah, D.O. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 186, 110681. [Google Scholar] [CrossRef] [PubMed]
- Argenta, D.F.; Bidone, J.; Koester, L.S.; Bassani, V.L.; Simoes, C.M.O.; Teixeira, H.F. Topical Delivery of Coumestrol from Lipid Nanoemulsions Thickened with Hydroxyethylcellulose for Antiherpes Treatment. AAPS PharmSciTech 2018, 19, 192–200. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; de Holanda e Silva, K.G.; Elias Mansur, C.R. Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater Sci. Eng. C 2018, 92, 245–253. [Google Scholar] [CrossRef]
- Kaci, M.; Belhaffef, A.; Meziane, S.; Dostert, G.; Menu, P.; Velot, E.; Desobry, S.; Arab-Tehrany, E. Nanoemulsions and topical creams for the safe and effective delivery of lipophilic antioxidant coenzyme Q10. Colloids Surf. B Biointerfaces 2018, 167, 165–175. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; de Holanda e Silva, K.G.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Sci. 2017, 116, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Miastkowska, M.; Sikora, E.; Ogonowski, J.; Zielina, M.; Ludzik, A. The kinetic study of isotretinoin release from nanoemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 63–68. [Google Scholar] [CrossRef]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Betzler de Oliveira de Siqueira, L.; da Silva Cardoso, V.; Rodrigues, I.A.; Vazquez-Villa, A.L.; Pereira dos Santos, E.; da Costa Leal Ribeiro Guimaraes, B.; dos Santos Cerqueira Coutinho, C.; Vermelho, A.B.; Ricci, E., Jr. Development and evaluation of zinc phthalocyanine nanoemulsions for use in photodynamic therapy for Leishmania spp. Nanotechnology 2017, 28, 65101. [Google Scholar] [CrossRef] [PubMed]
- Topical Drug Delivery Market Research Report by Product (Liquid Formulations, Semi-Solid Formulations, and Solid Formulations), by Rout to Administration (Dermal Drug Delivery, Nasal Drug Delivery, and Ophthalmic Drug Delivery), by End-User, by Region (Americas, Asia-Pacific, and Europe, Middle East & Africa)—Global Forecast to 2026—Cumulative Impact of COVID-19. In Global Topical Drug Delivery Industry; GLOBE NEWSWIRE: New York, NY, USA, 2021; Available online: https://www.reportlinker.com/p06033143/?utm_source=GNW (accessed on 19 October 2021).
- Proksch, E.; Berardesca, E.; Misery, L.; Engblom, J.; Bouwstra, J. Dry skin management: Practical approach in light of latest research on skin structure and function. J. Dermatolog. Treat. 2020, 31, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.A.E.; et al. Topical and cutaneous delivery using nanosystems. J. Control Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic Study to Determine the Structural Similarities between Artificial Membrane Strat-M™ and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Buheazaha, T.M.; AlHomoud, H.S.; Al-Nasif, H.A.; Sarafroz, M. A comparative ex vivo permeation evaluation of a novel 5-Fluorocuracil nanoemulsion-gel by topically applied in the different excised rat, goat, and cow skin. Saudi J. Biol. Sci. 2020, 27, 1024–1040. [Google Scholar] [CrossRef]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]
- Eiras, F.; Amaral, M.H.; Silva, R.; Martins, E.; Sousa Lobo, J.M.; Silva, A.C. Characterization and biocompatibility evaluation of cutaneous formulations containing lipid nanoparticles. Int. J. Pharm. 2017, 519, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Katiyar, S.S.; Kushwah, V.; Jain, S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin. Drug Deliv. 2017, 14, 165–177. [Google Scholar] [CrossRef] [PubMed]


| Drug Substance | Main Action | References |
|---|---|---|
| Corticosteroids | For local application. Manifests a slight immunosuppressive effect. Ineffectiveness in severe cases. | [49] |
| Retinoids | Retinoids can be synthetic or natural derivatives of vitamin A. Probable mechanisms of action: Facilitate the transport of cytoplasmic retinoid-binding proteins; influence angiogenesis; modulate T cell responses. | [50,51] |
| Vitamin D3 metabolites | Metabolites of vitamin D3 are included in ointments and creams for the treatment of psoriasis. Good effects in milder diseases. Not fully understood effects. The 1,25(OH)2D3 enhances the suppressive activity of CD4(+)CD25(+) cells in draining lymph nodes. | [52,53,54] |
| UVB treatment | Multiple effects and effectiveness for several T cell-mediated skin diseases. Not equally effective in the different disorders. Unspecific and generally immunosuppressive. | [55,56,57] |
| Methotrexate | Immunosuppressive effect. It does not target specific T cell groups. Still not a fully understood therapeutic effect. | [49,51] |
| Cyclosporine A | Affects IL-2 producing cells, in particular CD4(+) T cells. General immunosuppressive effect. | [49,51] |
| Lipid-Based Delivery System | Description | Advantages | Disadvantages |
|---|---|---|---|
| Nanovesicular carriers | |||
| Liposomes [68] | Conventional single or multilayer vesicles. Formed by contact of biodegradable lipids with an aqueous medium. Widely used as drug carriers for hydrophilic and lipophilic molecules. | Biocompatible and biodegradable lipids. Conventional production processes. Improved local delivery. Suitable for loading both hydrophobic and hydrophilic substances. | Insufficient chemical and physical stability. Short half-life. Inadequate penetration into the viable epidermis and dermis. High production costs. Difficulties in scalability. |
| Transfersomes [69,70,71] | Highly deformable, elastic or ultra-flexible liposomes. Vesicles, similar to conventional liposomes in terms of preparation and structure. Claimed to permeate as intact vesicles through the skin layers. Functionally deformed due to the presence of an edge activator. | Smaller vesicle size, higher elasticity. Compared with conventional liposomes—better penetration through the skin. High membrane hydrophilicity and elasticity allow them to avoid aggregation and fusion under osmotic stress, unlike the conventional liposomes. | Elasticity of these vesicles can be compromised by hydrophobic drug loading. Occlusive application and complete skin hydration limit transdermal delivery due to inhibition of transdermal hydration. Relatively high production costs. Absence of well-established regulatory guidance for skin delivery. |
| Ethosomes [72,73] | Lipid vesicles are composed of phospholipids, ethanol, and water. Similar to liposomes in terms of their preparation techniques and structure. Concentration of ethanol 20–45%. Their size decreases with an increase in the ethanol concentration. Exhibit high encapsulation efficiency. | Appropriate for both hydrophobic and hydrophilic drug loading. Enhanced skin delivery under both occlusive and nonocclusive conditions. Higher elasticity, smaller vesicle size, and higher entrapment efficiency than conventional liposomes. | High ethanol content can lead to skin irritation and toxicity. Possible structural and chemical instability during long-term storage. Need to optimize the concentration of lipids and ethanol for improved physicochemical properties and stability of ethosomes. |
| Lipid nanoparticulate carriers | |||
| Solid lipid nanoparticles [74,75] | Colloidal lipid nanoparticles are composed of a physiological biodegradable solid lipophilic matrix (solid at room temperature and body temperature), in which the drug molecules can be incorporated. | Increased drug stability. High drug payload. Incorporation of lipophilic and hydrophilic drugs. Avoidance of organic solvents. Lack of biotoxicity of the carrier. Relatively cost-effective. | SLN are incorporated into semisolid carriers such as ointments and gels due to the high water content. Potential expulsion of active compounds during storage. Cost-effective manufacturing process. |
| Nanostructure Lipid Carriers [76,77] | Colloidal lipid nanoparticles composed of physiological mixing liquid lipid (oils) with the solid lipids, in which the liquid lipid is incorporated into the solid matrix or localized at the surface of solid particles | Improved drug loading compared with SLN. Lower water content compared with SLN. Firmly incorporates the drug substance during storage. Biodegradable and biocompatible. Large-scale production is easily possible. | Tendency to unpredictable gelation. Polymorphic transition. Low drug incorporation due to the crystalline structure of solid lipids. Lack of long-term stability data. |
| Lipospheres [78,79,80] | Microspheres, composed of solid hydrophobic lipid core and stabilized by a monolayer of a phospholipid embedded on the surface. | Improved drug stability, especially for photo-labile drugs. Possibility for controlled drug release. Controlled particle size. High drug loading. Biodegradable and biocompatible. | Larger particle size and poor skin permeation compared with lipid-based vesicular carriers, SLN, and NLC. Poor drug loading for hydrophilic compounds. |
| Application | Title/Inventors | Year | Results |
|---|---|---|---|
| CN103006562 (A) | Daptomycin ethosome preparation/ Li Chong, Liu Xia, Yin Qikun, Wang Xiaoying, Chen Zhangbao | 2013 | Stable translucent dispersion system with a small and uniform particle size. High entrapment efficiency. Excellent transdermal performance. Simple and convenient preparation method. |
| EP 2810642 A1 | Chitosan-modified ethosome structure/ Chin-Tung Lee, Po-Liang Chen | 2013 | The chitosan-modified ethosome structure contains different active substances. Improved storage and transportation. |
| CN103800277 (A) | Leflunomide ethosome composition and its preparation method/ Zhang Tao, Ding Yanji, Deng Jie, Luo Jing, Zhong Xiaodong | 2014 | Improves the transdermal rate of leflunomide. Improves curative effects. |
| CN103536700 A | Chinese medicinal ethosome gel patch for treating herpes zoster and preparation method thereof/Bu Ping, Hu Rong, Chen Lin, Wei Rong, Wu Huanhuan, Huang Xiaoli | 2014 | Easy in medication. Convenient to use. Good therapeutic effect. Strong analgesic action. No adverse reaction. |
| CN 104706571 A | Preparation method of ethosome/ natural material/polyvinyl alcohol composite hydrogel/Yang Xingxing, Lynn, Chen Mengxia, Fanlin Peng | 2015 | Addition of the polyvinyl alcohol, which improves the properties of the hydrogel. |
| CN106474065A | A kind of tetracaine ethosome and its preparation technology/Zhu Xiaoliang, Wu Dongze, Ma Xiaodong | 2017 | Stable in terms of component and proportion. Preferable percutaneous permeation. |
| Emerging Lipid Vesicles | Description | Reference |
|---|---|---|
| Niosomes | Nonionic surfactant and cholesterol (or its derivatives)—based vesicle with improved stability (especially oxidative stability). | [105,106] |
| Cubosomes | Submicron, nanostructured particles, composed of bicontinuous cubic liquid crystalline phase. | [107,108,109] |
| Hexosomes | Constructed of hexagonal liquid crystalline phases dispersed in a continuous aqueous medium. | [110] |
| Aquasomes | Self-assembled nanovesicles, composed of three layers. | [111] |
| Colloidosomes | Hollow shell microcapsules composed of coagulated particles. | [112] |
| Sphingosomes | Contained sphingolipids such as sphingosine, ceramide, sphingomyelin or glycosphingolipid; and are concentric, bilayered nanovesicles with an acidic pH inside. | [113] |
| Ufasomes | Lipid carriers attach to the surface of the skin and support the lipid exchange between the outermost layers of the SC. | [114,115] |
| Archeosomes | Consisted of archebacteria lipids, chemically distinct from eukaryotic and prokaryotic species. Less sensitive to high temperature, alkaline pH, and oxidative stress. | [116,117] |
| Lipoplexes | Cationic lipid-DNA complexes. Efficient carriers for cell transfection. Toxic effects arising from either cationic lipids or nucleic acids. | [118] |
| Proliposomes | Dry, free-flowing particles that immediately form a liposomal dispersion in contact with water. | [119,120] |
| LNP Type | API/Drug | Application | Reference |
|---|---|---|---|
| Conventional liposomes | Licorice | Licorice-loaded liposomes included in the formulation for the treatment of oxidative stress injuries. | [146] |
| Conventional liposomes | Quercetin and resveratrol | Quercetin- and resveratrol-loaded liposomes for the treatment of inflammatory/oxidative responses associated with skin cancer. | [147] |
| Liposomes | Tretinoin | A tretinoin-loaded liposomal formulation for the treatment of acne. | [148] |
| Liposomes | Benzoyl peroxide | Benzoyl peroxide and chloramphenicol encapsulation in liposomes for the treatment of acne. | [149] |
| Liposomes | Benzoyl peroxide/Adapalene | Benzoyl peroxide- and adapalene-loaded modified liposomal gel for the treatment of acne. | [150] |
| Transfersomes | Indocyanine green | Indocyanine green-loaded transfersomes for the treatment of acne vulgaris. | [151] |
| Transfersomes | 5-Fluorouracil | 5-Fluorouracil-loaded transfersomes for the treatment of skin cancer. | [152] |
| Transfersomes | Resveratrol and 5-fluorouracil | Transfersomes containing resveratrol and 5-fluorouracil for the treatment of skin cancer. | [145] |
| Transfersomes | Amphotericin B | Development of amphotericin B-loaded transfersomes for antifungal and antileishmanial treatment. | [133] |
| Transfersomes | siRNA | Transfersomes containing siRNA developed for delivery to the human basal epidermis for the treatment of melanoma. | [153] |
| Transfersomes | RNAi | Transfersomes containing RNAi, formulated for the treatment of psoriasis. | [154] |
| Transfersomes | Indocyanine | Indocyanine-loaded transfersomes for the treatment of basal cell carcinoma. | [155] |
| Transfersomes | Clindamycin | Development of clindamycin-loaded transfersomes for the treatment of acne. | [156] |
| Transfersomes | Paclitaxel | Paclitaxel containing transfersomes, modified by a cell-penetrating-peptide embedded in oligopeptide hydrogel for the topical treatment of melanoma. | [157] |
| Transfersomes | Sodium stibogluconate | Transfersomes loaded with sodium stibogluconate for the treatment of leishmaniasis. | [158] |
| Transfersomes | Lidocaine | Lidocaine transferosomal gel, containing permeation enhancers for local anesthetic action. | [159] |
| Transfersomes | Sulforaphane | Transfersomes comprising sulforaphane for the treatment of skin cancer. | [160] |
| Transfersomes | Miltefosine polyphenol | Formulation of miltefosine polyphenol-loaded transfersomes for the topical treatment of leishmaniasis. | [161] |
| Transfersome | N-acetylcysteine | N-acetylcysteine-loaded transfersomes for antioxidant activity in anti-aging therapy. | [162] |
| Ethosomes | Methoxsalen | Formulation of ethosomes containing methoxsalen for the topical treatment against vitiligo. | [134] |
| Ethosomes | Griseofulvin | Design of griseofulvin-loaded ethosomes for enhanced antifungal treatment. | [163] |
| Ethosomes | Cryptotanshinone | Cryptotanshinone-loaded ethosomes for anti-acne treatment. | [164] |
| Ethosomes | Epigallocatechin-3-gallate | Epigallocatechin-3-gallate-loaded ethosomes for the treatment of skin cancer. | [165] |
| Ethosomes | Thymoquinone | Thymoquinone-loaded ethosomes for the topical treatment of acne. | [166] |
| Ethosomes | Clobetasol propionate | Ethosomes of clobetasol propionate for the treatment of eczema. | [167] |
| Ethosomes | Tretinoin | Gel containing tretinoin-loaded ethosomes for anti-acne treatment. | [168] |
| Ethosomes | Azelaic acid | Azelaic acid-loaded ethosomes for anti-acne treatment. | [137] |
| Niosomes | Resveratrol | Resveratrol-loaded niosomes for the treatment of psoriasis. | [169] |
| Niosomes | Diacerein | Niosomes for the topical diacerein delivery and treatment of psoriasis. | [170] |
| Niosomes | Celastrol | Celastrol-loaded niosomes for the treatment of psoriasis. | [171] |
| Cubosomes | Paclitaxel | Paclitaxel-loaded cubosomes against skin cancer. | [172] |
| Cubosomes | Erythromycin | Erythromycin-loaded cubosomes for the treatment of acne. | [173] |
| Hexosomes, cubosomes | Ketoconazole | Ketoconazole-loaded hexosomes for antifungal treatment. | [174] |
| Ufasomes | Minoxidil | Minoxidil-loaded ufasomes for the treatment of hair loss. | [175] |
| LNP Type | API/Drug | Application | Reference |
|---|---|---|---|
| SLN | Doxorubicin | Doxorubicin-loaded SLN for the treatment of skin cancer. | [206] |
| SLN | Adapalene | Adapalene-loaded SLN in the gel for anti-acne treatment. | [207] |
| SLN | Triamcinolone acetonide | Triamcinolone acetonide-loaded SLN for the topical treatment of psoriasis. | [208] |
| SLN | Resveratrol, vitamin E, and epigallocatechin gallate | SLN containing resveratrol, vitamin E, and epigallocatechin gallate for antioxidant benefits. | [209] |
| SLN | Silybin | Silybin-loaded SLN enriched gel for irritant contact dermatitis. | [210] |
| SLN | Fluconazole | Fluconazole-loaded SLN topical gel for the treatment of pityriasis versicolor. | [211] |
| SLN | Tazarotene | Tazarotene-loaded SLN for the treatment of psoriasis. | [212] |
| SLN | Miconazole nitrate | Miconazole nitrate-loaded SLN for antifungal activity. | [213] |
| SLN | Adapalene | Adapalene-loaded SLN for anti-acne therapy. | [214] |
| SLN | Isotretinoin and α-tocopherol | SLN loaded with retinoic acid and lauric acid for the topical treatment of acne vulgaris. | [215] |
| NLC | Spironolactone | Spironolactone-loaded NLC-based gel for the effective treatment of acne vulgaris. | [216] |
| NLC | Clobetasol propionate | NLC-based topical gel of clobetasol propionate for the treatment of eczema. | [217] |
| NLC | Tacrolimus and tumor necrosis factor α siRNA | NLC co-delivering tacrolimus and tumor necrosis factor α siRNA for the treatment of psoriasis. | [218] |
| NLC | Itraconazole | Topical NLC containing itraconazole for the treatment of fungal infections. | [219] |
| NLC | Apremilast | NLC for topical delivery of apremilast for the treatment of psoriasis. | [220] |
| NLC | Dithranol | Dithranol-loaded NLC-based gel for the treatment of psoriasis. | [221] |
| NLC | Voriconazole | Voriconazole-loaded NLC for antifungal applications. | [222] |
| NLC | Mometasone furoate | NLC-based hydrogel of mometasone furoate for the treatment of psoriasis. | [223] |
| NLC | Antimicrobial peptide nisin Z | Antimicrobial peptide nisin Z with conventional antibiotic-loaded NLC to enhance antimicrobial activity. | [224] |
| NLC | Adapalene and vitamin C | Adapalene- and vitamin C-loaded NLC for acne treatment. | [225] |
| Type | API/Drug | Application | Reference |
|---|---|---|---|
| Microemulsion | Tazarotene | Tazarotene-loaded microemulsion for the treatment of psoriasis. | [244] |
| Microemulsion | Methotrexate | Methotrexate-loaded microemulsion for the treatment of psoriasis. | [245] |
| Microemulsion | Retinoid | Retinoid-loaded microemulsion for the treatment of psoriasis. | [246] |
| Microemulsion | Clotrimazole | Microemulsion coated with chitosan and containing clotrimazole for antifungal activity. | [247] |
| Microemulsion | Griseofulvin | Griseofulvin-loaded microemulsion for the antifungal treatment. | [248] |
| Microemulsion | Boswellia carterii oleo-gum-resin | Boswellia carterii oleo-gum resin-loaded microemulsion for the treatment of acne and eczema. | [249] |
| Microemulsion | Indian pennywort, walnut, and turmeric | Topical dosage microemulsion of Indian pennywort, walnut, and turmeric for the treatment of eczema. | [250] |
| Microemulsion | Triamcinolone | Microemulsion containing triamcinolone for transdermal delivery for the treatment of eczema. | [251] |
| Microemulsion | Retinyl palmitate | Microemulsion containing retinyl palmitate for the treatment of acne, aging, and psoriasis. | [252] |
| Nanoemulsion | Triptolide | Triptolide nanoemulsion gels for the treatment of eczema. | [253] |
| Nanoemulsion | Ivermectin | Nanoemulsion containing ivermectin for the treatment of different types of parasite infestations. | [254] |
| Nanoemulsion | Cyclosporine | Cyclosporine-loaded nanoemulsion for the treatment of psoriasis. | [255] |
| Nanoemulsion | Coumestrol /Hydroxyethylcellulose | Nanoemulsion containing coumestrol and hydroxyethylcellulose for the treatment of antiherpes. | [256] |
| Nanoemulsion | 8-Methoxypsoralen | 8-Methoxypsoralenloaded nanoemulsion for the treatment of vitiligo and psoriasis. | [257] |
| Nanoemulsion | Coenzyme Q10 | Coenzyme Q10-loaded nanoemulsion as an antioxidant agent. | [258] |
| Nanoemulsion | Psoralen | Psoralen-loaded nanoemulsion for the treatment of psoriasis and vitiligo. | [259] |
| Nanoemulsion | Isotretinoin | Isotretinoin-loaded nanoemulsion for the treatment of acne. | [260] |
| Nanoemulsion | Amphotericin B | Amphotericin B-loaded nanoemulsion for the antifungal treatment. | [261] |
| Nanoemulsion | Zinc phthalocyanine | Zinc phthalocyanine-loaded nanoemulsion for use in photodynamic therapy for leishmaniasis. | [262] |
| Type | API/Drug | Disease | Reference |
|---|---|---|---|
| Nanoemulsion gel | Clobetasol propionate | Treatments of psoriasis. | [266] |
| Nanoethogel | Amphotericin B | Dermatophytes and fungal infections. | [236] |
| Nanoemulsion gel | 5-Fluorouracil | Non-melanoma skin cancers. | [267] |
| Hydrogel | Zinc oxide | Wound healing effect on fibroblast cells. | [268] |
| Nanoemulgel, NLC | Vitamin E | Skin hydration. | [269] |
| Hydrogel | Cyclosporine and calcipotriol | Treatments of psoriasis. | [270] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanov, S.R.; Andonova, V.Y. Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders. Pharmaceuticals 2021, 14, 1083. https://doi.org/10.3390/ph14111083
Stefanov SR, Andonova VY. Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders. Pharmaceuticals. 2021; 14(11):1083. https://doi.org/10.3390/ph14111083
Chicago/Turabian StyleStefanov, Stefan R., and Velichka Y. Andonova. 2021. "Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders" Pharmaceuticals 14, no. 11: 1083. https://doi.org/10.3390/ph14111083
APA StyleStefanov, S. R., & Andonova, V. Y. (2021). Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders. Pharmaceuticals, 14(11), 1083. https://doi.org/10.3390/ph14111083