Anti-Aging β-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells
Abstract
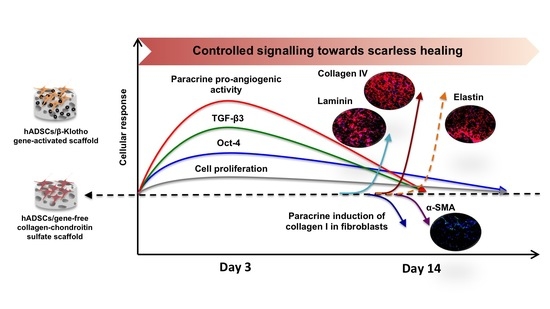
:1. Introduction
2. Results
2.1. β-Klotho Gene-Activated Scaffold Transiently Enhances Human ADSCs’ Stemness and Pro-Reparative Genes
2.2. β-Klotho Gene-Activated Scaffold Enhances the Paracrine Potency of Human ADSCs
2.2.1. Pro-Angiogenic Bioactivity
2.2.2. Dermal Fibroblast Healing and Maturation
2.3. β-Klotho Gene-Activated Scaffold Enhances Basement Membrane Regeneration with Improved Anti-Fibrotic Response in Human ADSCs
3. Discussion
4. Materials and Methods
4.1. Preparation of Gene-Activated Scaffold
4.2. Cell Seeding on β-Klotho Gene-Activated Scaffold
4.3. qRT-PCR Analyses to Determine β-Klotho Gene Overexpression and Activation of Functional Genes
4.4. Bioactivity Analyses of Secreted Factors from the ADSCs on β-Klotho Gene-Activated Scaffold
4.4.1. Pro-Angiogenic Bioactivity Analyses
4.4.2. Dermal Fibroblasts Healing and Maturation Analyses
4.5. Immunofluorescence Imaging of Extracellular Matrix Proteins
Image Quantification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J. Tissue Eng. Regen. Med. 2018, 12, e296–e312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makrantonaki, E.; Wlaschek, M.; Scharffetter-Kochanek, K. Pathogenesis of wound healing disorders in the elderly. JDDG J. Dtsch. Dermatol. Ges. 2017, 15, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The analysis of in vivo aging in human bone marrow mesenchymal stromal cells using colony-forming unit-fibroblast assay and the CD45lowCD271+ phenotype. Stem Cells Int. 2019, 2019, 5197983. [Google Scholar] [CrossRef] [Green Version]
- Westerweel, P.E.; Teraa, M.; Rafii, S.; Jaspers, J.E.; White, I.A.; Hooper, A.T.; Verhaar, M.C. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS ONE 2013, 8, e60357. [Google Scholar] [CrossRef] [Green Version]
- Murray, R.Z.; West, Z.E.; Cowin, A.J.; Farrugia, B.L. Development and use of biomaterials as wound healing therapies. Burn. Trauma 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimian, T.G.; Pouzoulet, F.; Squiban, C.; Buard, V.; André, M.; Cousin, B.; Tamarat, R. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foubert, P.; Gonzalez, A.D.; Teodosescu, S.; Berard, F.; Doyle-Eisele, M.; Yekkala, K.; Fraser, J.K. Adipose-Derived Regenerative Cell Therapy for Burn Wound Healing: A Comparison of Two Delivery Methods. Adv. Wound Care 2016, 5, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assi, R.; Foster, T.R.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Dardik, A. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen. Med. 2016, 11, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Chen, B.; Liu, Y.; Zhufu, Z.; Yan, X.; Hou, X.; Tan, Q. Effect of collagen scaffold with adipose-derived stromal vascular fraction cells on diabetic wound healing: A study in a diabetic porcine model. Tissue Eng. Regen. Med. 2013, 10, 192–199. [Google Scholar] [CrossRef]
- Falanga, V.; Sabolinski, M. A bilayered living skin construct (APLIGRAF®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999, 7, 201–207. [Google Scholar] [CrossRef]
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the treatment of chronic wounds. Adv. Wound Care. 2012, 1, 138–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, T.L.; Veves, A. The efficacy of Apligraf in the treatment of diabetic foot ulcers. Plast. Reconstr. Surg. 2006, 117, 152S–157S. [Google Scholar] [CrossRef] [PubMed]
- Eaglstein, W.H.; Falanga, V. Tissue engineering and the development of Apligraf®, a human skin equivalent. Clin. Ther. 1997, 19, 894–905. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, G.; Wang, Q.; Yang, L.; Zheng, L.; Zhao, J.; Zhang, X. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis 2017, 8, e2851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, X.; Li, Z.; Lendlein, A.; Ma, N. Genetic engineering of mesenchymal stem cells by non-viral gene delivery. Clin. Hemorheol. Microcirc. 2014, 58, 19–48. [Google Scholar] [CrossRef] [Green Version]
- Deveza, L.; Choi, J.; Lee, J.; Huang, N.; Cooke, J.; Yang, F. Polymer-DNA Nanoparticle-Induced CXCR4 Overexpression Improves Stem Cell Engraftment and Tissue Regeneration in a Mouse Hindlimb Ischemia Model. Theranostics 2016, 6, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Yannas, I.; Tzeranis, D.; Harley, B.; So, P. Biologically active collagen-based scaffolds: Advances in processing and characterization. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2123–2139. [Google Scholar] [CrossRef] [Green Version]
- Laiva, A.L.; Raftery, R.M.; Keogh, M.B.; O’Brien, F.J. Pro-angiogenic impact of SDF-1α gene-activated collagen-based scaffolds in stem cell driven angiogenesis. Int. J. Pharm. 2018, 544, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Lackington, W.A.; Raftery, R.M.; O’Brien, F.J. In vitro efficacy of a gene-activated nerve guidance conduit incorporating non-viral PEI-pDNA nanoparticles carrying genes encoding for NGF, GDNF and c-Jun. Acta Biomater. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Tierney, E.G.; Duffy, G.P.; Hibbitts, A.J.; Cryan, S.A.; O’Brien, F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control. Release 2012, 158, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1α gene-activated collagen scaffold drives functional differentiation of human Schwann cells for wound healing applications. Biotechnol. Bioeng. 2021, 118, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1α gene-activated collagen scaffold enhances provasculogenic response in a coculture of human endothelial cells with human adipose-derived stromal cells. J. Mater. Sci. Mater. Med. 2021, 32, 26. [Google Scholar] [CrossRef]
- Bonadio, J.; Smiley, E.; Patil, P.; Goldstein, S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat Med 1999, 5, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Kolakshyapati, P.; Li, X.; Chen, C.; Zhang, M.; Tan, W.; Ma, L.; Gao, C. Gene-activated matrix/bone marrow-derived mesenchymal stem cells constructs regenerate sweat glands-like structure in vivo. Sci. Rep. 2017, 7, 17630. [Google Scholar] [CrossRef] [Green Version]
- Marolt, D.; Knezevic, M.; Vunjak-Novakovic, G. Bone tissue engineering with human stem cells. Stem Cell Res. Ther. 2010, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Holm, J.S.; Toyserkani, N.M.; Sorensen, J.A. Adipose-derived stem cells for treatment of chronic ulcers: Current status. Stem Cell Res. Ther. 2018, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1α Gene-Activated Collagen Scaffold Restores Pro-Angiogenic Wound Healing Features in Human Diabetic Adipose-Derived Stem Cells. Biomedicines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Wan Safwani, W.K.; Makpol, S.; Sathapan, S.; Chua, K.H. The changes of stemness biomarkers expression in human adipose-derived stem cells during long-term manipulation. Biotechnol. Appl. Biochem. 2011, 58, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Suku, M.; Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Anti-Ageing Protein β-Klotho Rejuvenates Diabetic Stem Cells for Improved Gene-Activated Scaffold Based Wound Healing. J. Pers. Med. 2020, 11, 4. [Google Scholar] [CrossRef]
- Ullah, M.; Sun, Z. Klotho Deficiency Accelerates Stem Cells Aging by Impairing Telomerase Activity. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Sun, Z. The Antiaging Gene Klotho Regulates Proliferation and Differentiation of Adipose-Derived Stem Cells. Stem Cells 2016, 34, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Li, Y.; Liu, Y.; Shao, L.; Yu, J.; Li, Z. Overexpression of klotho in adipose-derived stem cells protects against UVB-induced photoaging in co-cultured human fibroblasts. Mol. Med. Rep. 2018, 18, 5473–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wan, X.; Cao, Y.Z.; Sun, D.; Cao, C.C. Klotho gene-modified BMSCs showed elevated antifibrotic effects by inhibiting the Wnt/β-catenin pathway in kidneys after acute injury. Cell Biol. Int. 2018, 42, 1670–1679. [Google Scholar] [CrossRef]
- Branski, L.; Pereira, C.; Herndon, D.; Jeschke, M. Gene therapy in wound healing: Present status and future directions. Gene Ther. 2007, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, P.; Basma, H.; Klinkebiel, D.; Christman, J.; Cheng, P.-W. Cell type-specific activation of the cytomegalovirus promoter by dimethylsulfoxide and 5-aza-2’-deoxycytidine. Int. J. Biochem. Cell Biol. 2008, 40, 1944–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Zhang, H.; Li, Y.; Wang, N.; Zhang, W.; Yang, K.; Wu, N.; Chen, X.; Deng, F.; Liao, Z.; et al. Characterization of constitutive promoters for piggyBac transposon-mediated stable transgene expression in mesenchymal stem cells (MSCs). PLoS ONE 2014, 9, e94397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardee, C.L.; Arévalo-Soliz, L.M.; Hornstein, B.D.; Zechiedrich, L. Advances in non-viral DNA vectors for gene therapy. Genes 2017, 8, 65. [Google Scholar] [CrossRef]
- Alhaji, S.Y.; Ngai, S.C.; Abdullah, S. Silencing of transgene expression in mammalian cells by DNA methylation and histone modifications in gene therapy perspective. Biotechnol. Genet. Eng. Rev. 2019, 35, 1–25. [Google Scholar] [CrossRef]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellison, D.D.; Suhail, Y.; Afzal, J.; Woo, L.; Kilic, O.; Spees, J.; Levchenko, A. Dynamic secretome of bone marrow-derived stromal cells reveals a cardioprotective biochemical cocktail. Proc. Natl. Acad. Sci. USA 2019, 116, 14374–14383. [Google Scholar]
- Shi, G.; Jin, Y. Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res. Ther. 2010, 1, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.-M.; Han, S.-H.; Coh, Y.-R.; Jang, G.; Ra, J.C.; Kang, S.-K.; Lee, H.-W.; Youn, H.-Y. Enhanced proliferation and differentiation of Oct4-and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp. Mol. Med. 2014, 46, e101. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Landau, S.; Flugelman, M.Y.; Levenberg, S. Genetically engineered human muscle transplant enhances murine host neovascularization and myogenesis. Commun. Biol. 2018, 1, 161. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Kornhaber, R.; Jaeger, M.; Harats, M.; Aviv, U.; Zerach, A.; Haik, J. Treatment of hypergranulation tissue in burn wounds with topical steroid dressings: A case series. Int. Med. Case Rep. J. 2016, 9, 241–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, A.; Baiju, I.; Bhat, I.A.; Pandey, S.; Bharti, M.; Verma, M.; Singh, A.P.; Ansari, M.M.; Chandra, V.; Saikumar, G.; et al. Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. J. Cell. Physiol. 2020, 235, 5555–5569. [Google Scholar] [CrossRef]
- Ni, C.-W.; Kumar, S.; Ankeny, C.J.; Jo, H. Development of immortalized mouse aortic endothelial cell lines. Vasc. Cell 2014, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, J.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Brauer, E.; Lippens, E.; Klein, O.; Nebrich, G.; Schreivogel, S.; Korus, G.; Duda, G.N.; Petersen, A. Collagen Fibrils Mechanically Contribute to Tissue Contraction in an In Vitro Wound Healing Scenario. Adv. Sci. 2019, 6, 1801780. [Google Scholar] [CrossRef]
- Berry, D.P.; Harding, K.G.; Stanton, M.R.; Jasani, B.; Ehrlich, H.P. Human wound contraction: Collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr. Surg. 1998, 102, 124–131. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, W.; Gao, J.; Liu, J.; Wang, H.; Li, J.; Yang, X.; He, T.; Guan, H.; Zheng, Z.; et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res. Ther. 2016, 7, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ma, Y.; Gao, Z.; Yang, J. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem Cell Res. Ther. 2018, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, G.; Rittié, L. Restoration of the basement membrane after wounding: A hallmark of young human skin altered with aging. J. Cell Commun. Signal. 2018, 12, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenester, E.; Yurchenco, P.D. Laminins in basement membrane assembly. Cell Adhes. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Idrees, M.; Joo, M.-D.; Sidrat, T.; Wei, Y.; Song, S.-H.; Lee, K.-L.; Kong, I.-K. Constitutive Expression of TERT Enhances β-Klotho Expression and Improves Age-Related Deterioration in Early Bovine Embryos. Int. J. Mol. Sci. 2021, 22, 5327. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Uehara, Y.; Motomura-Matsuzaka, K.; Oki, J.; Koyama, Y.; Kimura, M.; Asada, M.; Komi-Kuramochi, A.; Oka, S.; Toru, T. βKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 2008, 22, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Y.; Schéele, S.; Arman, E.; Haffner-Krausz, R.; Ekblom, P.; Lonai, P. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J. Cell Biol. 2001, 153, 811–822. [Google Scholar] [CrossRef] [Green Version]
- LeBleu, V.S.; MacDonald, B.; Kalluri, R. Structure and function of basement membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef]
- Roig-Rosello, E.; Rousselle, P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, Y.; Geng, Z.; Ma, K.; Sun, X.; Fu, X. Abnormalities in the basement membrane structure promote basal keratinocytes in the epidermis of hypertrophic scars to adopt a proliferative phenotype. Int. J. Mol. Med. 2016, 37, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Chen, Y.; Chai, M.; Tao, R.; Lei, Y.; Jia, Y.; Shu, J.; Ren, J.; Li, G.; Wei, W. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose-derived stem cells into fibroblasts. Int. J. Mol. Med. 2019, 43, 890–900. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, N.J.; Lee, D.D.H.; Gowers, K.H.; Butler, C.R.; Maughan, E.F.; Jevans, B.; Orr, J.C.; McCann, C.J.; Burns, A.J.; MacNeil, S.; et al. Bioengineered airway epithelial grafts with mucociliary function based on collagen IV-and laminin-containing extracellular matrix scaffolds. Eur. Respir. J. 2020, 55, 1901200. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Chen, L.; Bond, J.E.; Medina, M.A.; Ren, L.; Kokosis, G.; Selim, A.M.; Levinson, H. Myofibroblasts contribute to but are not necessary for wound contraction. Lab. Investig. 2015, 95, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Liu, L.N.; Yong, Q.; Deng, J.C.; Cao, W.G. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther 2015, 6, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizaki, A.; Yanaba, K.; Yoshizaki, A.; Iwata, Y.; Komura, K.; Ogawa, F.; Takenaka, M.; Shimizu, K.; Asano, Y.; Hasegawa, M.; et al. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 2010, 62, 2476–2487. [Google Scholar] [CrossRef]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin signaling in wound repair. Birth Defects Res. C Embryo Today 2012, 96, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]





| Function | Primer (Catalog No.) | Encoded Gene |
|---|---|---|
| Activation and proliferation |
Hs_KLB_4_SG (QT02454977) | Beta Klotho (β-Klotho) |
|
Hs_MKI67_1_SG (QT00014203) | Marker of proliferation (Ki-67) | |
| Stemness or pluripotency promoters | Hs_POU5 F1_1_SG (QT00210840) | Octamer-binding transcription factor 4 (Oct-4) |
| Hs_NANOG_1_SG (QT01025850) | Homeobox protein (Nanog) | |
| Hs_SOX2_1_SG (QT00237601) | Sex determining region Y-box 2 (Sox-2) | |
| Wound healing regulators | Hs_TGFB3_1_SG (QT00001302) | Transforming growth factor beta 3 (TGF-β3) |
| Hs_TGFB1_1_SG (QT00000728) | Transforming growth factor beta 1 (TGF-β1) |
| Functional Roles | Primary Antibodies (Catalog No.) | Dilutions in 1% BSA Solution |
|---|---|---|
| Basement membrane proteins | Fibronectin (ab2413, Abcam, UK) | 1:200 |
| Laminin (ab11575, Abcam, UK) | 1:200 | |
| Collagen IV (ab6586, Abcam, UK) | 1:200 | |
| Scar-associated contractile protein | Alpha-smooth muscle actin (ab7817, Abcam, UK) | 1:100 |
| Elastic matrix protein | Elastin (ab21607, Abcam, UK) | 1:200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Anti-Aging β-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells. Pharmaceuticals 2021, 14, 1168. https://doi.org/10.3390/ph14111168
Laiva AL, O’Brien FJ, Keogh MB. Anti-Aging β-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells. Pharmaceuticals. 2021; 14(11):1168. https://doi.org/10.3390/ph14111168
Chicago/Turabian StyleLaiva, Ashang L., Fergal J. O’Brien, and Michael B. Keogh. 2021. "Anti-Aging β-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells" Pharmaceuticals 14, no. 11: 1168. https://doi.org/10.3390/ph14111168
APA StyleLaiva, A. L., O’Brien, F. J., & Keogh, M. B. (2021). Anti-Aging β-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells. Pharmaceuticals, 14(11), 1168. https://doi.org/10.3390/ph14111168