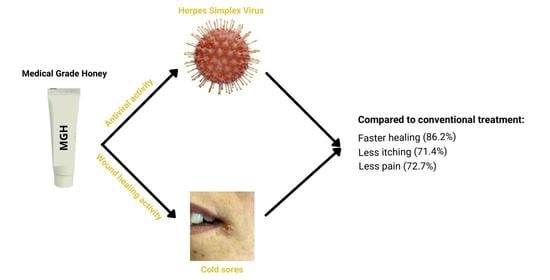
Medical-Grade Honey Outperforms Conventional Treatments for Healing Cold Sores—A Clinical Study
Abstract
:1. Introduction
2. Results
2.1. Healing Time Is Lower with MGH Compared to Conventional Treatment
2.2. Pain Is Lower with MGH Than Conventional Treatment
2.3. Less Itching with MGH Than Conventional Treatment
2.4. Patients Strongly Prefer MGH over Conventional Therapies
3. Discussion
4. Materials and Methods
4.1. Design of the Study
4.2. Patient Population
4.3. Treatment
4.4. Outcome Parameters
4.5. Ethical Statement
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [Green Version]
- Looker, K.J.; Welton, N.J.; Sabin, K.M.; Dalal, S.; Vickerman, P.; Turner, K.M.E.; Boily, M.C.; Gottlieb, S.L. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: A population attributable fraction analysis using published epidemiological data. Lancet Infect. Dis. 2020, 20, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Opstelten, W.; Neven, A.K.; Eekhof, J. Treatment and prevention of herpes labialis. Can. Fam. Physician 2008, 54, 1683–1687. [Google Scholar]
- Ashwini Rani, S.R.; Suragimath, G.; Rajmane, V.; Rajmane, Y. Prevalence of recurrent herpes labialis in Western Maharashtra. J. Oral. Maxillofac. Pathol. 2021, 25, 51–54. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Chemaitelly, H.; Abu-Raddad, L.J. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: Model-based predictions. BMC Med. 2019, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Cernik, C.; Gallina, K.; Brodell, R.T. The treatment of herpes simplex infections: An evidence-based review. Arch. Intern. Med. 2008, 168, 1137–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, C.C.; Wang, S.H.; Delamere, F.M.; Wojnarowska, F.; Peters, M.C.; Kanjirath, P.P. Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database Syst. Rev. 2015, 8, CD010095. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Guttman-Yassky, E. Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J. Allergy Clin. Immunol. 2014, 134, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amir, J.; Harel, L.; Smetana, Z.; Varsano, I. Treatment of herpes simplex gingivostomatitis with aciclovir in children: A randomised double blind placebo controlled study. BMJ 1997, 314, 1800–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moin, A.T.; Chowdhury, M.-b.; Riana, S.H.; Ullah, M.d.A.; Araf, Y.; Sarkar, B.; Shohael, A.M. An Updated Overview of Herpes Simplex Virus-1 Infection: Insights from Origin to Mitigation Measures. Electron. J. Gen. Med. 2021, 18, em299. [Google Scholar]
- Armour, M.; Semprini, A.; Ee, C.; MacCullagh, L.; Shortt, N. Efficacy of a topical herbal and mineral formulation (Dynamiclear) for the treatment of herpes simplex labialis in the community setting: Study protocol for a randomised, double-blind placebo-controlled trial. BMJ Open 2020, 10, e031876. [Google Scholar] [CrossRef] [Green Version]
- Valacyclovir Description. Available online: https://www.mayoclinic.org/drugs-supplements/valacyclovir-oral-route/side-effects/drg-20066635?p=1 (accessed on 15 October 2021).
- Available online: https://www.drugs.com/acyclovir.html (accessed on 15 October 2021).
- Available online: https://www.singlecare.com/blog/acyclovir-vs-valacyclovir/ (accessed on 15 October 2021).
- Kumar, M.; Hill, J.M.; Clement, C.; Varnell, E.D.; Thompson, H.W.; Kaufman, H.E. A double-blind placebo-controlled study to evaluate valacyclovir alone and with aspirin for asymptomatic HSV-1 DNA shedding in human tears and saliva. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5601–5608. [Google Scholar] [CrossRef] [Green Version]
- Karadi, I.; Karpati, S.; Romics, L. Aspirin in the management of recurrent herpes simplex virus infection. Ann. Intern. Med. 1998, 128, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, F.; Kiani, A.; Fateh, M. Aspirin in the management of recurrent herpes simplex virus infection: A randomized blinded, controlled crossover trial. J. Res. Med. Sci. 2003, 8, 51–53. [Google Scholar]
- Crimi, S.; Fiorillo, L.; Bianchi, A.; D’Amico, C.; Amoroso, G.; Gorassini, F.; Mastroieni, R.; Marino, S.; Scoglio, C.; Catalano, F.; et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses 2019, 11, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef] [PubMed]
- Israili, Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef]
- Shahzad, A.; Cohrs, R.J. In vitro antiviral activity of honey against varicella zoster virus (VZV): A translational medicine study for potential remedy for shingles. Transl. Biomed. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Rahmasari, R.; Matsunaga, A.; Haruyama, T.; Kobayashi, N. Anti-influenza viral effects of honey in vitro: Potent high activity of manuka honey. Arch. Med. Res. 2014, 45, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Ghapanchi, J.; Moattari, A.; Andisheh Tadbir, A.; Talatof, Z.; Pour Shahidi, S.; Ebrahimi, H. The In Vitro Anti-Viral Activity of Honey on Type 1 Herpes Simplex Virus. Aust. J. Basic Appl. Sci. 2011, 5, 849–852. [Google Scholar]
- Abuelgasim, H.; Albury, C.; Lee, J. Effectiveness of honey for symptomatic relief in upper respiratory tract infections: A systematic review and meta-analysis. BMJ Evid. Based Med. 2020, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Ghasemi, S.; Farkhondeh, T.; Azimi-Nezhad, M.; Shakibaei, M.; Samarghandian, S. Possible Potential Effects of Honey and Its Main Components Against Covid-19 Infection. Dose Response 2021, 19, 1559325820982423. [Google Scholar] [CrossRef]
- Mustafa, M.Z.; Shamsuddin, S.H.; Sulaiman, S.A.; Abdullah, J.M. Anti-inflammatory Properties of Stingless Bee Honey May Reduce the Severity of Pulmonary Manifestations in COVID-19 Infections. Malays. J. Med. Sci. 2020, 27, 165–169. [Google Scholar] [CrossRef]
- Hossain, K.S.; Hossain, M.G.; Moni, A.; Rahman, M.M.; Rahman, U.H.; Alam, M.; Kundu, S.; Rahman, M.M.; Hannan, M.A.; Uddin, M.J. Prospects of honey in fighting against COVID-19: Pharmacological insights and therapeutic promises. Heliyon 2020, 6, e05798. [Google Scholar] [CrossRef]
- Ashraf, S.; Ashraf, M.; Imran, M.A.; Kalsoom, L.; Siddiqui, U.; Farooq, I.; Habib, Z.; Ashraf, S.; Ghufran, M.; Akram, M.K.; et al. Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multi-center placebo-controlled randomized clinical trial. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Nair, H.K.R.; Tatavilis, N.; Pospisilova, I.; Kucerova, J.; Cremers, N.A.J. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- de Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.J.; Chowdhary, A.; Meis, J.F. Antifungal Activity of a Medical-Grade Honey Formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Cremers, N.A.J.; Leeming, J.P.; van der Werf, E.T. Sweet Relief: Determining the Antimicrobial Activity of Medical Grade Honey Against Vaginal Isolates of Candida albicans. J. Fungi 2019, 5, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.M.P.; Devesa, J.S.P.; Hill, P.B. In vitro efficacy of a honey-based gel against canine clinical isolates of Staphylococcus pseudintermedius and Malassezia pachydermatis. Vet. Dermatol. 2018, 29, 180-e165. [Google Scholar] [CrossRef] [PubMed]
- Pleeging, C.C.F.; Coenye, T.; Mossialos, D.; De Rooster, H.; Chrysostomou, D.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 2020, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Hashemipour, M.A.; Tavakolineghad, Z.; Arabzadeh, S.A.; Iranmanesh, Z.; Nassab, S.A. Antiviral Activities of Honey, Royal Jelly, and Acyclovir Against HSV-1. Wounds 2014, 26, 47–54. [Google Scholar] [PubMed]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef] [Green Version]
- Gaby, A.R. Natural remedies for Herpes simplex. Altern. Med. Rev. 2006, 11, 93–101. [Google Scholar] [PubMed]
- Uozaki, M.; Ikeda, K.; Tsujimoto, K.; Nishide, M.; Yamasaki, H.; Khamsri, B.; Koyama, A.H. Antiviral effects of dehydroascorbic acid. Exp. Ther. Med. 2010, 1, 983–986. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Holubova, A.; Chlupacova, L.; Cetlova, L.; Cremers, N.A.J.; Pokorna, A. Medical-Grade Honey as an Alternative Treatment for Antibiotics in Non-Healing Wounds-A Prospective Case Series. Antibiotics 2021, 10, 918. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. Medical grade honey for the treatment of extravasation-induced injuries in preterm neonates—A case series. Adv. Neonatal Care 2021, 21, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Smaropoulos, E.; Cremers, N.A. Medical grade honey for the treatment of paediatric abdominal wounds: A case series. J. Wound Care 2020, 29, 94–99. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. The pro-healing effects of medical grade honey supported by a pediatric case series. Complement. Ther. Med. 2019, 45, 14–18. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. Treating severe wounds in pediatrics with medical grade honey: A case series. Clin. Case Rep. 2020, 8, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marais, H.J.; Glyphis, Z.G.; Cremers, N.A.J. Medical grade honey: Hope for wounded white rhinos. Vet. Anim. Sci. 2021, 13, 100196. [Google Scholar] [CrossRef] [PubMed]
- Willihnganz, M.J.; Gurevitz, S.L.; Clayton, B.D. Clayton's Basic Pharmacology for Nurses—E-Book, 18th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Semprini, A.; Singer, J.; Braithwaite, I.; Shortt, N.; Thayabaran, D.; McConnell, M.; Weatherall, M.; Beasley, R. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: A randomised controlled trial. BMJ Open 2019, 9, e026201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Waili, N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004, 10, MT94–MT98. [Google Scholar]
- Abdel-Naby Awad, A.G.; Hamad, A.-M.H. Honey can help in herpes simplex gingivostomatitis in children: Prospective randomized double blind placebo controlled clinical trial. Am. J. Otoryngol. 2018, 39, 759–763. [Google Scholar] [CrossRef]
- Liu, T.M.; Luo, Y.W.; Tam, K.W.; Lin, C.C.; Huang, T.W. Prophylactic and therapeutic effects of honey on radiochemotherapy-induced mucositis: A meta-analysis of randomized controlled trials. Support Care Cancer 2019, 27, 2361–2370. [Google Scholar] [CrossRef]
- Munstedt, K.; Momm, F.; Hubner, J. Honey in the management of side effects of radiotherapy- or radio/chemotherapy-induced oral mucositis. A systematic review. Complement Ther. Clin. Pract. 2019, 34, 145–152. [Google Scholar] [CrossRef]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet. Dermatol. 2020, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Spruance, S.L.; Hamill, M.L.; Hoge, W.S.; Davis, L.G.; Mills, J. Acyclovir prevents reactivation of herpes simplex labialis in skiers. JAMA 1988, 260, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, K.; Tatz, A.M.; Slavin, R.A.; Dahan, R.; Ahmad, W.A.; Sutton, G.A.; Kelmer, G. Will local intraoperative application of Medical Grade Honey in the incision protect against incisional infection in horses undergoing colic surgery? AAEP Proc. 2019, 65, 387–388. [Google Scholar]
- Mandel, H.H.; Sutton, G.A.; Abu, E.; Kelmer, G. Intralesional application of medical grade honey improves healing of surgically treated lacerations in horses. Equine Vet. J. 2020, 52, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangold, C.A.; Rathbun, M.M.; Renner, D.W.; Kuny, C.V.; Szpara, M.L. Viral infection of human neurons triggers strain-specific differences in host neuronal and viral transcriptomes. PLoS Pathog. 2021, 17, e1009441. [Google Scholar] [CrossRef] [PubMed]



| Number of Patients Evaluated (% of Cohort) | Faster Healing with MGH Number (%) | Similar Healing Number (%) | Slower Healing with MGH Number (%) | Significant Different Compared to Conventional Treatment | |
|---|---|---|---|---|---|
| Objective healing time | 29 (100) | 25 (86.2) | 2 (6.9) | 2 (6.9) | p < 0.005 |
| Subjective healing experience | 29 (100) | 23 (79.3) | 6 (20.7) | 0 (0) | p < 0.005 |
| Number of Patients Experiencing Pain (% of Cohort) | Less Pain with MGH Number (%) | Similar Pain Number (%) | More Pain with MGH Number (%) | Significance | |
|---|---|---|---|---|---|
| MGH treatment compared to conventional treatment | 22 (75.9) | 16 (72.7) | 6 (27.3) | 0 (0) | p < 0.005 |
| Number of Patients Experiencing Itching (% of Cohort) | Less Itching with MGH Number (%) | Similar Itching Number (%) | More Itching with MGH Number (%) | Significance | |
|---|---|---|---|---|---|
| MGH treatment compared to conventional treatment | 21 (72.4) | 15 (71.4) | 6 (28.6) | 0 (0) | p < 0.005 |
| Frequency | Quite Exceptionally (about 1 Time per Year) | Occasionally (2–3 Times per Year) | Often (4–5 Times per Year) | Very Often (>5 Times per Year) |
|---|---|---|---|---|
| Number of patients (total 29) | 3 | 7 | 10 | 9 |
| Percentage | 10.3% | 24.1% | 34.5% | 31.0% |
| Age Group | 18–26 Years | 27–35 Years | 36–44 Years | 45–53 Years | 54–65 Years | >65 Years |
|---|---|---|---|---|---|---|
| Number of patients (total 29) | 8 | 5 | 10 | 3 | 2 | 1 |
| Percentage | 27.6% | 17.2% | 34.5% | 10.3% | 6.9% | 3.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, P.P.; Mossialos, D.; Wijk, B.v.; Novakova, P.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Medical-Grade Honey Outperforms Conventional Treatments for Healing Cold Sores—A Clinical Study. Pharmaceuticals 2021, 14, 1264. https://doi.org/10.3390/ph14121264
Naik PP, Mossialos D, Wijk Bv, Novakova P, Wagener FADTG, Cremers NAJ. Medical-Grade Honey Outperforms Conventional Treatments for Healing Cold Sores—A Clinical Study. Pharmaceuticals. 2021; 14(12):1264. https://doi.org/10.3390/ph14121264
Chicago/Turabian StyleNaik, Piyu Parth, Dimitris Mossialos, Bas van Wijk, Petra Novakova, Frank A. D. T. G. Wagener, and Niels A. J. Cremers. 2021. "Medical-Grade Honey Outperforms Conventional Treatments for Healing Cold Sores—A Clinical Study" Pharmaceuticals 14, no. 12: 1264. https://doi.org/10.3390/ph14121264
APA StyleNaik, P. P., Mossialos, D., Wijk, B. v., Novakova, P., Wagener, F. A. D. T. G., & Cremers, N. A. J. (2021). Medical-Grade Honey Outperforms Conventional Treatments for Healing Cold Sores—A Clinical Study. Pharmaceuticals, 14(12), 1264. https://doi.org/10.3390/ph14121264