Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-kB Inflammatory Axis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Chemical Agents and Medication
4.3. Inducing Type 1 Diabetes Mellitus in the Male Mice
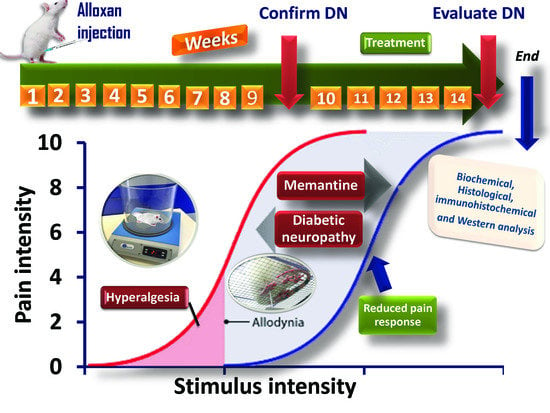
4.4. Experimental Design
4.5. Behavioral Assessment of Pain Responses
4.5.1. Measurement of Thermal Hyperalgesia
4.5.2. Measurement of Mechanical Allodynia
4.6. Sacrification of Mice and Organ Dissection
4.7. Histopathological and Immunohistochemical Assays
4.8. Immunohistochemical Assessment of NF-kB Protein
4.9. Electron Microscopy
4.10. Assessment of Spinal Glutamate and Cytokine Levels
4.11. Western Blotting Analysis for Spinal NMDAR1, HMGB1, TLR4, and p-NF-kB
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochoa, J.L. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2009, 72, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Bril, V.; England, J.; Franklin, G.M.; Backonja, M.; Cohen, J.; Del Toro, D.; Feldman, E.; Iverson, D.J.; Perkins, B.; Russell, J.W.; et al. Evidence-based Guideline: Treatment of Painful Diabetic Neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PMR 2011, 3, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Brod, M.; Pohlman, B.; Blum, S.I.; Ramasamy, A.; Carson, R. Burden of Illness of Diabetic Peripheral Neuropathic Pain: A Qualitative Study. Patient Patient-Cent. Outcomes Res. 2014, 8, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Obrosova, I.G. Diabetic painful and insensate neuropathy: Pathogenesis and potential treatments. Neurotherapeutics 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.-D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [Green Version]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Prim. 2017, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Morioka, N.; Miyauchi, K.; Miyashita, K.; Kochi, T.; Zhang, F.F.; Nakamura, Y.; Liu, K.; Wake, H.; Hisaoka-Nakashima, K.; Nishibori, M. Spinal high-mobility group box-1 induces long-lasting mechanical hypersensitivity through the toll-like receptor 4 and upregulation of interleukin-1β in activated astrocytes. J. Neurochem. 2019, 150, 738–758. [Google Scholar] [CrossRef]
- Maeda, T.; Ozaki, M.; Kobayashi, Y.; Kiguchi, N.; Kishioka, S. HMGB1 as a Potential Therapeutic Target for Neuropathic Pain. J. Pharmacol. Sci. 2013, 123, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Pedrazzi, M.; Averna, M.; Sparatore, B.; Patrone, M.; Salamino, F.; Marcoli, M.; Maura, G.; Cervetto, C.; Frattaroli, D.; Pontremoli, S.; et al. Potentiation of NMDA Receptor-Dependent Cell Responses by Extracellular High Mobility Group Box 1 Protein. PLoS ONE 2012, 7, e44518. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Cao, L.; Khanabdali, R.; Kalionis, B.; Tai, X.; Xia, S. The Emerging Role of HMGB1 in Neuropathic Pain: A Potential Therapeutic Target for Neuroinflammation. J. Immunol. Res. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Balosso, S.; Liu, J.; Bianchi, M.E.; Vezzani, A. Disulfide-Containing High Mobility Group Box-1 PromotesN-Methyl-d-Aspartate Receptor Function and Excitotoxicity by Activating Toll-Like Receptor 4-Dependent Signaling in Hippocampal Neurons. Antioxid. Redox Signal. 2014, 21, 1726–1740. [Google Scholar] [CrossRef]
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-analysis. J. Alzheimer’s Dis. 2017, 60, 401–425. [Google Scholar] [CrossRef]
- Rajasekar, N.; Nath, C.; Hanif, K.; Shukla, R. Inhibitory Effect of Memantine on Streptozotocin-Induced Insulin Receptor Dysfunction, Neuroinflammation, Amyloidogenesis, and Neurotrophic Factor Decline in Astrocytes. Mol. Neurobiol. 2015, 53, 6730–6744. [Google Scholar] [CrossRef]
- Cheng, Q.; Fang, L.; Feng, D.; Tang, S.; Yue, S.; Huang, Y.; Han, J.; Lan, J.; Liu, W.; Gao, L.; et al. Memantine ameliorates pulmonary inflammation in a mice model of COPD induced by cigarette smoke combined with LPS. Biomed. Pharmacother. 2019, 109, 2005–2013. [Google Scholar] [CrossRef]
- Sevostianova, N.; Danysz, W.; Bespalov, A.Y. Analgesic effects of morphine and loperamide in the rat formalin test: Interactions with NMDA receptor antagonists. Eur. J. Pharmacol. 2005, 525, 83–90. [Google Scholar] [CrossRef]
- Sang, C.N.; Booher, S.; Gilron, I.; Parada, S.; Max, M.B. Dextromethorphan and Memantine in Painful Diabetic Neuropathy and Postherpetic NeuralgiaEfficacy and Dose-Response Trials. J. Am. Soc. Anesthesiol. 2002, 96, 1053–1061. [Google Scholar] [CrossRef]
- Rogers, M.; Rasheed, A.; Moradimehr, A.; Baumrucker, S.J. Memantine (Namenda) for Neuropathic Pain. Am. J. Hosp. Palliat. Med. 2008, 26, 57–59. [Google Scholar] [CrossRef]
- Nikolajsen, L.; Gottrup, H.; Kristensen, A.G.; Jensen, T.S. Memantine (a N-methyl-D-aspartate receptor antag-onist) in the treatment of neuropathic pain after amputation or surgery: A randomized, double-blinded, cross-over study. Anesth. Analg. 2000, 91, 960–966. [Google Scholar] [CrossRef]
- Fashner, J.; Bell, A.L. Herpes zoster and postherpetic neuralgia: Prevention and management. Am. Fam. Physician 2011, 83, 1432–1437. [Google Scholar]
- McCormick, Z.; Chang-Chien, D.G.; Marshall, D.B.; Huang, M.; Harden, R.N. Phantom Limb Pain: A Systematic Neuroanatomical-Based Review of Pharmacologic Treatment. Pain Med. 2014, 15, 292–305. [Google Scholar] [CrossRef]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, M.K.; Helmy, S.A.; Badran, D.I.; Zaitone, S.A. Neuroprotective effect of duloxetine in a mouse model of diabetic neuropathy: Role of glia suppressing mechanisms. Life Sci. 2018, 205, 113–124. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Ahmed, E.; Kader, G.A.; Abdel-Mottaleb, Y.; ElSayed, M.H.; Youssef, A.M.; Zaitone, S.A. In-hibitory effect of valproate sodium on pain behavior in diabetic mice involves suppression of spinal histone deacetylase 1 and inflammatory mediators. Int. Immunopharmacol. 2019, 70, 16–27. [Google Scholar] [CrossRef]
- El-Sherbeeny, N.A.; Ibrahiem, A.T.; Ali, H.S.; Farag, N.E.; Toraih, E.A.; Zaitone, S.A. Carbamazepine conquers spinal GAP43 deficiency and sciatic Nav1.5 upregulation in diabetic mice: Novel mechanisms in alleviating allodynia and hyperalgesia. Arch. Pharmacal Res. 2020, 43, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.R.-R.; Nackley, P.A.; Huh, B.Y.; Terrando, P.N.; Maixner, D.W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-R.; Samoriski, G.; Pan, H.-L. Antinociceptive effects of chronic administration of uncompetitive NMDA receptor antagonists in a rat model of diabetic neuropathic pain. Neuropharmacology 2009, 57, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correll, G.E.; Maleki, J.; Gracely, E.J.; Muir, J.J.; Harbut, R.E. Subanesthetic ketamine infusion therapy: A ret-rospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004, 5, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Cvrček, P. Side Effects of Ketamine in the Long-Term Treatment of Neuropathic Pain. Pain Med. 2008, 9, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K.; Coderre, T.J.; Hagen, N.A. Targeting the N-methyl-D-aspartate receptor for chronic pain manage-ment: Preclinical animal studies, recent clinical experience and future research directions. J. Pain Symptom Manag. 2000, 20, 358–373. [Google Scholar] [CrossRef]
- Recla, J.M.; Sarantopoulos, C.D. Combined use of pregabalin and memantine in fibromyalgia syndrome treatment: A novel analgesic and neuroprotective strategy? Med. Hypotheses 2009, 73, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Weng, H.-R. Endogenous Interleukin-1β in Neuropathic Rats Enhances Glutamate Release from the Primary Afferents in the Spinal Dorsal Horn through Coupling with Presynaptic N-Methyl-d-aspartic Acid Receptors. J. Biol. Chem. 2013, 288, 30544–30557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.J.; Salter, M.W. Glutamate receptor phosphorylation and trafficking in pain plasticity in spinal cord dorsal horn. Eur. J. Neurosci. 2010, 32, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hulsebosch, C.E.; Leem, J.W. Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury. Neural Plast. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Weyerbacher, A.R.; Xu, Q.; Tamasdan, C.; Shin, S.J.; Inturrisi, C.E. N -Methyl-d-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain 2010, 148, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Garraway, S.M.; Xu, Q.; Inturrisi, C.E. siRNA-Mediated Knockdown of the NR1 Subunit Gene of the NMDA Receptor Attenuates Formalin-Induced Pain Behaviors in Adult Rats. J. Pain 2009, 10, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Olivares, D.; Deshpande, V.K.; Shi, Y.; Lahiri, D.K.; Greig, N.H.; Rogers, J.T.; Huang, X. N-Methyl D-Aspartate (NMDA) Receptor Antagonists and Memantine Treatment for Alzheimer’s Disease, Vascular Dementia and Parkinson’s Disease. Curr. Alzheimer Res. 2012, 9, 746–758. [Google Scholar] [CrossRef]
- Suzuki, R.; Matthews, E.A.; Dickenson, A.H. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain 2001, 91, 101–109. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Malmberg, A.B.; Yaksh, T.L. Efficacy of spinal NMDA receptor antagonism in formalin hyper-algesia and nerve injury evoked allodynia in the rat. J. Pharmacol. Exp. Ther. 1997, 280, 829–838. [Google Scholar]
- Carlton, S.M.; Rees, H.; Tsuruoka, M.; Willis, W.D. Memantine attenuates responses of spinothalamic tract cells to cutaneous stimulation in neuropathic monkeys. Eur. J. Pain 1998, 2, 229–238. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Semple, B.D.; Jones, N.C.; Othman, I.; Shaikh, M.F. High mobility group box 1 (HMGB1) as a novel frontier in epileptogenesis: From pathogenesis to therapeutic approaches. J. Neurochem. 2019, 151, 542–557. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.; Sadanandan, J.; Chattopadhyay, M. High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy. Int. J. Mol. Sci. 2020, 21, 881. [Google Scholar] [CrossRef] [Green Version]
- Elshaer, R.E.; Tawfik, M.K.; Nosseir, N.; El-Ghaiesh, S.H.; Toraih, E.A.; Elsherbiny, N.M.; Zaitone, S.A. Leflunomide-induced liver injury in mice: Involvement of TLR4 mediated activation of PI3K/mTOR/NFκB pathway. Life Sci. 2019, 235, 116824. [Google Scholar] [CrossRef]
- Huang, X.-T.; Li, C.; Peng, X.-P.; Guo, J.; Yue, S.-J.; Liu, W.; Zhao, F.-Y.; Han, J.-Z.; Huang, Y.-H.; Yang-Li, Y.-L.; et al. An excessive increase in glutamate contributes to glucose-toxicity in β-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Sung, C.S.; Wen, Z.H.; Feng, C.W.; Chen, C.H.; Huang, S.Y.; Chen, N.F.; Chen, W.F.; Wong, C.S. Potentiation of spinal glutamatergic response in the neuron-glia interactions underlies the intrathecal IL-1β-induced thermal hyperal-gesia in rats. CNS Neurosci. Ther. 2017, 23, 580–589. [Google Scholar] [CrossRef]
- Kikumoto, Y.; Sugiyama, H.; Inoue, T.; Morinaga, H.; Takiue, K.; Kitagawa, M.; Fukuoka, N.; Saeki, M.; Maeshima, Y.; Wang, D.-H.; et al. Sensitization to alloxan-induced diabetes and pancreatic cell apoptosis in acatalasemic mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Elsherbiny, N.M.; Abdel-Mottaleb, Y.; Elkazaz, A.Y.; Atef, H.; Lashine, R.M.; Youssef, A.M.; Ezzat, W.; El-Ghaiesh, S.H.; Elshaer, R.E.; El-Shafey, M.; et al. Carbamazepine Alleviates Retinal and Optic Nerve Neural Degeneration in Diabetic Mice via Nerve Growth Factor-Induced PI3K/Akt/mTOR Activation. Front. Neurosci. 2019, 13, 1089. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Tang, Y.; Zhao, J.; Li, Q.; Fan, X.; Xu, H.; Yin, Z.Q. Neuroprotective effect of memantine on the retinal ganglion cells of APPswe/PS1ΔE9 mice and its immunomodulatory mechanisms. Exp. Eye Res. 2015, 135, 47–58. [Google Scholar] [CrossRef]
- Higgs, J.; Wasowski, C.; Loscalzo, L.M.; Marder, M. In vitro binding affinities of a series of flavonoids for μ-opioid receptors. Antinociceptive effect of the synthetic flavonoid 3,3-dibromoflavanone in mice. Neuropharmacology 2013, 72, 9–19. [Google Scholar] [CrossRef]
- Reda, H.M.; Zaitone, S.A.; Moustafa, Y.M. Effect of levetiracetam versus gabapentin on peripheral neuropathy and sciatic degeneration in streptozotocin-diabetic mice: Influence on spinal microglia and astrocytes. Eur. J. Pharmacol. 2016, 771, 162–172. [Google Scholar] [CrossRef]
- Chaplan, S.; Bach, F.; Pogrel, J.; Chung, J.; Yaksh, T. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Yalcin, I.; Benbouzid, M.; Tessier, L.-H.; Müller, A.; Hein, L.; Barrot, M.; Choucair-Jaafar, N.; Freund-Mercier, M.-J. β2-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann. Neurol. 2009, 65, 218–225. [Google Scholar] [CrossRef]
- Ko, M.J.; Mulia, G.E.; Van Rijn, R.M. Commonly Used Anesthesia/Euthanasia Methods for Brain Collection Differentially Impact MAPK Activity in Male and Female C57BL/6 Mice. Front. Cell. Neurosci. 2019, 13, 96. [Google Scholar] [CrossRef]
- Greish, S.; Abogresha, N.; Zaitone, S. Duloxetine Modulates Vincristine-Induced Painful Neuropathy in Rats. J. Physiol. Pharmacol. Adv. 2014, 4, 420–430. [Google Scholar] [CrossRef]
- Eaton, S.E.; Harris, N.D.; Rajbhandari, S.M.; Greenwood, P.; Wilkinson, I.D.; Ward, J.D.; Griffiths, P.D.; Tesfaye, S. Spinal-cord involvement in diabetic peripheral neuropathy. Lancet 2001, 358, 35–36. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high PH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.K. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- El-Ghaiesh, S.H.; Bahr, H.I.; Ibrahiem, A.T.; Ghorab, D.; AlOmar, S.Y.; Farag, N.E.; Zaitone, S.A. Metformin Protects from Rotenone–Induced Nigrostriatal Neuronal Death in Adult Mice by Activating AMPK-FOXO3 Signaling and Mitigation of Angiogenesis. Front. Mol. Neurosci. 2020, 13, 84. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomar, S.Y.; Gheit, R.E.A.E.; Enan, E.T.; El-Bayoumi, K.S.; Shoaeir, M.Z.; Elkazaz, A.Y.; Al Thagfan, S.S.; Zaitone, S.A.; El-Sayed, R.M. Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-kB Inflammatory Axis. Pharmaceuticals 2021, 14, 307. https://doi.org/10.3390/ph14040307
Alomar SY, Gheit REAE, Enan ET, El-Bayoumi KS, Shoaeir MZ, Elkazaz AY, Al Thagfan SS, Zaitone SA, El-Sayed RM. Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-kB Inflammatory Axis. Pharmaceuticals. 2021; 14(4):307. https://doi.org/10.3390/ph14040307
Chicago/Turabian StyleAlomar, Suliman Y., Rehab E. Abo El Gheit, Eman T. Enan, Khaled S. El-Bayoumi, Mohamed Z. Shoaeir, Amany Y. Elkazaz, Sultan S. Al Thagfan, Sawsan A. Zaitone, and Rehab M. El-Sayed. 2021. "Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-kB Inflammatory Axis" Pharmaceuticals 14, no. 4: 307. https://doi.org/10.3390/ph14040307
APA StyleAlomar, S. Y., Gheit, R. E. A. E., Enan, E. T., El-Bayoumi, K. S., Shoaeir, M. Z., Elkazaz, A. Y., Al Thagfan, S. S., Zaitone, S. A., & El-Sayed, R. M. (2021). Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-kB Inflammatory Axis. Pharmaceuticals, 14(4), 307. https://doi.org/10.3390/ph14040307