3′,4′-Dihydroxyflavonol Modulates the Cell Cycle in Cancer Cells: Implication as a Potential Combination Drug in Osteosarcoma
Abstract
:1. Introduction
2. Results
2.1. DiOHF Inhibits Osteosarcoma Proliferation through Non-Apoptotic Mechanisms
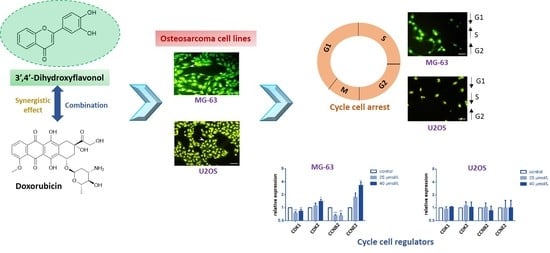
2.2. DiOHF Enhances the Efficacy of The Anthracycline Doxorubicin (Dox)
2.3. DiOHF Induces Cell Cycle Arrest and Modulates Cyclin and Cyclin-Dependent Kinase Expression In Vitro
2.4. DiOHF Inhibits Nuclear Division in Cytokinesis-Blocked Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture Media and Reagents
4.2. Cell Culture and Exposure Conditions
4.3. Cytotoxicity Assays
4.4. Drug Combination Analysis
4.5. Cell Apoptosis and Necrosis
4.6. Cell Cycle Analysis
4.7. Cytokinesis-Block Micronucleus (CBMN) Cytome Assay
4.8. RNA Extraction, Reverse Transcription and qPCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 1–10. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed. Eng. Online 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of osteosarcoma and current management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [Green Version]
- Gazouli, I.; Kyriazoglou, A.; Kotsantis, I.; Anastasiou, M.; Pantazopoulos, A.; Prevezanou, M.; Chatzidakis, I.; Kavourakis, G.; Economopoulou, P.; Kontogeorgakos, V.; et al. Systematic review of recurrent osteosarcoma systemic therapy. Cancers 2021, 13, 1757. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Fanelli, M.; Tavanti, E.; Vella, S.; Ferrari, S.; Picci, P.; Serra, M. Advances in emerging drugs for osteosarcoma. Exp. Opin. Emerg. Drugs 2015, 20, 495–514. [Google Scholar] [CrossRef]
- Carvalho, F.S.; Burgeiro, A.; Garcia, R.; Moreno, A.J.; Carvalho, R.A.; Oliveira, P.J. Doxorubicin-induced cardiotoxicity: From bioenergetic failure and cell death to cardiomyopathy. Med. Res. Rev. 2014, 34, 106–135. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekins, S.; Williams, A.J.; Krasowski, M.D.; Freundlich, J.S. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov. Today 2011, 16, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Pantziarka, P.; Meheus, L. Omics-driven drug repurposing as a source of innovative therapies in rare cancers. Exp. Opin. Orphan Drugs 2018, 6, 513–517. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hagiwara, K.; Shirai, N.; Yoshida, K.; Hagiwara, H. Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology 2015, 67, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.-J.; Kim, S.-Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef]
- Léotoing, L.; Davicco, M.J.; Lebecque, P.; Wittrant, Y.; Coxam, V. The flavonoid fisetin promotes osteoblasts differentiation through Runx2 transcriptional activity. Mol. Nutr. Food Res. 2014, 58, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, W.; Tian, B.; Liu, X.; Qu, X.; Zhai, Z.; Li, H.; Liu, F.; Fan, Q.; Tang, T.; et al. Myricetin prevents titanium particle-induced osteolysis in vivo and inhibits RANKL-induced osteoclastogenesis in vitro. Biochem. Pharmacol. 2015, 93, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, Y.; Dong, Y.; Cheng, P.; Chen, A.; Huang, H. Flavonoids active against osteosarcoma: A review of the molecular mechanisms involved. Curr. Pharm. Des. 2017, 23, 1993–2001. [Google Scholar] [CrossRef]
- Chen, X.; Gu, N.; Xue, C.; Li, B.R. Plant flavonoid taxifolin inhibits the growth, migration and invasion of human osteosarcoma cells. Mol. Med. Rep. 2018, 17, 3239–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xu, X.; Jiang, T.; Wu, K.; Ding, C.; Liu, Z.; Zhang, X.; Yu, T.; Song, C. Citrus aurantium naringenin prevents osteosarcoma progression and recurrence in the patients who underwent osteosarcoma surgery by improving antioxidant capability. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed. Pharmacother. 2019, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, B.; Liu, W.; Wei, G.; Li, Z.; Yu, N.; Xue, X.; Ji, G. Acacetin induces apoptosis in human osteosarcoma cells by modulation of ROS/JNK activation. Drug Des. Devel. Ther. 2020, 14, 5077–5085. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Yan, J.; Liu, Y. Flavonoid compound breviscapine suppresses human osteosarcoma Saos-2 progression property and induces apoptosis by regulating mitochondria-dependent pathway. J. Biochem. Mol. Toxicol. 2021, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients. Food Chem. Toxicol. 2010, 48, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, X.; Xia, H. The flavonoid luteolin enhances doxorubicin-induced autophagy in human osteosarcoma U2OS cells. Int. J. Clin. Exp. Med. 2015, 8, 15190–15197. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodman, O.L.; Chan, E. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef]
- Dasdelen, D.; Solmaz, M.; Menevse, E.; Mogulkoc, R.; Baltaci, A.K.; Erdogan, E. Increased apoptosis, tumor necrosis factor-α, and DNA damage attenuated by 3’,4’-dihydroxyflavonol in rats with brain İschemia-reperfusion. Indian J. Pharmacol. 2021, 53, 39–49. [Google Scholar] [CrossRef]
- Yap, S.; Qin, C.; Woodman, O.L. Effects of resveratrol and flavonols on cardiovascular function: Physiological mechanisms. Biofactors 2010, 36, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Rationalizing combination therapies. Nat. Med. 2017, 23, 1113. [CrossRef] [PubMed] [Green Version]
- Song, M.J.; Baek, I.; Seo, M.; Kim, S.H.; Suk, K.; Woodman, O.L.; Williams, S.J.; Kim, I.K. Effects of 3’,4’-dihydroxyflavonol on vascular contractions of rat aortic rings. Clin. Exp. Pharmacol. Physiol. 2010, 37, 803–810. [Google Scholar] [CrossRef]
- Leo, C.-H.; Hart, J.L.; Woodman, O.L. 3′,4′-Dihydroxyflavonol reduces superoxide and improves nitric oxide function in diabetic rat mesenteric arteries. PLoS ONE 2011, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Couto, D.; Alves, A.; Dias, I.; Freitas, M.; Porto, G.; Duarte, J.A.; Fernandes, E. Trihydroxyflavones with antioxidant and anti-inflammatory efficacy. Biofactors 2012, 38, 378–386. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Fanelli, M.; Tavanti, E.; Vella, S.; Riganti, C.; Picci, P.; Serra, M. Doxorubicin-resistant osteosarcoma: Novel therapeutic approaches in sight? Future Oncol. 2017, 13, 673–677. [Google Scholar] [CrossRef]
- Prudowsky, Z.D.; Yustein, J.T. Recent insights into therapy resistance in osteosarcoma. Cancers 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, D.; Zhu, K. SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition. J. Exp. Clin. Cancer Res. 2018, 37, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Sramkoski, R.M.; Jacobberger, J.W. The kinetics of G2 and M transitions regulated by B cyclins. PLoS ONE 2013, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enserink, J.M.; Kolodner, R.D. An overview of Cdk1-controlled targets and processes. Cell Div. 2010, 5, 1–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catanzaro, D.; Ragazzi, E.; Vianello, C.; Caparrotta, L.; Montopoli, M. Effect of quercetin on cell cycle and cyclin expression in ovarian carcinoma and osteosarcoma cell lines. Nat. Prod. Commun. 2015, 10, 1365–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndt, K.; Campanile, C.; Muff, R.; Strehler, E.; Born, W.; Fuchs, B. Evaluation of quercetin as a potential drug in osteosarcoma treatment. Anticancer Res. 2013, 33, 1297–1306. [Google Scholar] [PubMed]
- Yang, Z.; Li, X.; Han, W.; Lu, X.; Jin, S.; Yang, W.; Li, J.; He, W.; Qian, Y. Galangin suppresses human osteosarcoma cells: An exploration of its underlying mechanism. Oncol. Rep. 2017, 37, 435–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagaki, N.; Sowa, Y.; Oki, T.; Nakanishi, R.; Yogosawa, S.; Sakai, T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int. J. Oncol. 2005, 26, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xie, M.; Jiang, N.; Huang, F.; Zhang, X.; Li, R.; Lu, J.; Liao, S.; Liu, Y. Icarisid II inhibits the proliferation of human osteosarcoma cells by inducing apoptosis and cell cycle arrest. Tumor Biol. 2017, 39, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, S.; Geng, B.; Yi, Z. Pelargonidin induces antitumor effects in human osteosarcoma cells via autophagy induction, loss of mitochondrial membrane potential, G2/M cell cycle arrest and downregulation of PI3K/AKT signalling pathway. J. BUON 2018, 23, 735–740. [Google Scholar] [PubMed]
- Peng, C.; Yang, Q.; Wei, B.; Yuan, B.; Liu, Y.; Li, Y.; Gu, D.; Yin, G.; Wang, B.; Xu, D.; et al. Investigation of crucial genes and microRNAs in conventional osteosarcoma using gene expression profiling analysis. Mol. Med. Rep. 2017, 16, 7617–7624. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Q.; Liu, D.; Li, X.; Deng, L.; Li, N.; Shen, J.; Zhao, Z.; Chen, J. CDC20 and its downstream genes: Potential prognosis factors of osteosarcoma. Int. J. Clin. Oncol. 2019, 24, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Liu, A.G.; Cai, K.T.; He, R.Q.; Li, Z.; Wei, Q.J.; Chen, M.Y.; Huang, J.Y.; Yan, W.Y.; Zhou, H.; et al. Exploration and validation of downregulated microRNA-199a-3p, downstream messenger RNA targets and transcriptional regulation in osteosarcoma. Am. J. Transl. Res. 2019, 11, 7538–7554. [Google Scholar]
- Wang, Y.; Kong, D. LncRNA GAS5 Represses osteosarcoma cells growth and metastasis via sponging MiR-203a. Cell. Physiol. Biochem. 2018, 45, 844–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Chen, C.C.; Shieh, T.M.; Hsueh, C.; Wang, S.H.; Leu, Y.L.; Lian, J.H.; Wang, T.H. Corylin suppresses hepatocellular carcinoma progression via the inhibition of epithelial-mesenchymal transition, mediated by long noncoding RNA GAS5. Int. J. Mol. Sci. 2018, 19, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Zhu, Y.; Chen, S.; Xu, R.; Wang, K. Fibroblast growth factor receptor 1 promotes MG63 cell proliferation and is associated with increased expression of cyclin-dependent kinase 1 in osteosarcoma. Mol. Med. Rep. 2016, 13, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Han, X.R.; Sun, Y.; Bai, X.Z. The anti-tumor role and mechanism of integrated and truncated PDCD5 proteins in osteosarcoma cells. Cell. Signal. 2012, 24, 1713–1721. [Google Scholar] [CrossRef]
- Lu, X.Y.; Lu, Y.; Zhao, Y.J.; Jaeweon, K.; Kang, J.; Xiao-Nan, L.; Ge, G.; Meyer, R.; Perlaky, L.; Hicks, J.; et al. Cell cycle regulator gene CDC5L, a potential target for 6p12-p21 amplicon in osteosarcoma. Mol. Cancer Res. 2008, 6, 937–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chang, H.; Gao, D.; Wang, L.; Jiang, N.; Yu, B. CDC5L contributes to malignant cell proliferation in human osteosarcoma via cell cycle regulation. Int. J. Clin. Exp. Pathol. 2016, 9, 10451–10457. [Google Scholar]
- Klimaszewska-Wisniewska, A.; Halas-Wisniewska, M.; Tadrowski, T.; Gagat, M.; Grzanka, D.; Grzanka, A. Paclitaxel and the dietary flavonoid fisetin: A synergistic combination that induces mitotic catastrophe and autophagic cell death in A549 non-small cell lung cancer cells. Cancer Cell Int. 2016, 16, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Salmela, A.L.; Pouwels, J.; Varis, A.; Kukkonen, A.M.; Toivonen, P.; Halonen, P.K.; Perälä, M.; Kallioniemi, O.; Gorbsky, G.J.; Kallio, M.J. Dietary flavonoid fisetin induces a forced exit from mitosis by targeting the mitotic spindle checkpoint. Carcinogenesis 2009, 30, 1032–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, L.; Oliveira, H.; Pacheco, A.R.; Almeida, L.; Pimentel, F.; Santos, C.; Ferreira de Oliveira, J.M. Hesperetin-etoposide combinations induce cytotoxicity in U2OS cells: Implications on therapeutic developments for osteosarcoma. DNA Repair 2017, 50, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| Cell Line | Mean IC50 (μmol/L) ± SD 1 | |||
|---|---|---|---|---|
| MTT 2 Assay | CV 3 Assay | |||
| DiOHF 4 | Dox 5 | DiOHF | Dox | |
| MG-63 | 103 ± 41 | 0.750 ± 0.101 | 50.3 ± 1.6 | 0.475 ± 0.136 |
| U2OS | 93.3 ± 19.8 | 0.434 ± 0.078 | 72.1 ± 12.3 | 0.454 ± 0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira de Oliveira, J.M.P.; Almeida, J.F.D.; Martins, M.; Proença, C.; Oliveira, H.; Fernandes, E.; Santos, C. 3′,4′-Dihydroxyflavonol Modulates the Cell Cycle in Cancer Cells: Implication as a Potential Combination Drug in Osteosarcoma. Pharmaceuticals 2021, 14, 640. https://doi.org/10.3390/ph14070640
Ferreira de Oliveira JMP, Almeida JFD, Martins M, Proença C, Oliveira H, Fernandes E, Santos C. 3′,4′-Dihydroxyflavonol Modulates the Cell Cycle in Cancer Cells: Implication as a Potential Combination Drug in Osteosarcoma. Pharmaceuticals. 2021; 14(7):640. https://doi.org/10.3390/ph14070640
Chicago/Turabian StyleFerreira de Oliveira, José Miguel P., Joana Filipa D. Almeida, Maria Martins, Carina Proença, Helena Oliveira, Eduarda Fernandes, and Conceição Santos. 2021. "3′,4′-Dihydroxyflavonol Modulates the Cell Cycle in Cancer Cells: Implication as a Potential Combination Drug in Osteosarcoma" Pharmaceuticals 14, no. 7: 640. https://doi.org/10.3390/ph14070640
APA StyleFerreira de Oliveira, J. M. P., Almeida, J. F. D., Martins, M., Proença, C., Oliveira, H., Fernandes, E., & Santos, C. (2021). 3′,4′-Dihydroxyflavonol Modulates the Cell Cycle in Cancer Cells: Implication as a Potential Combination Drug in Osteosarcoma. Pharmaceuticals, 14(7), 640. https://doi.org/10.3390/ph14070640