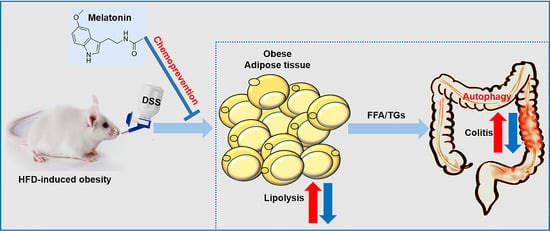
Melatonin Attenuates Dextran Sodium Sulfate Induced Colitis in Obese Mice
Abstract
:1. Introduction
2. Results
2.1. Obesity Aggravates Colitis Severity upon Dextran Sodium Sulfate (DSS) Challenge
2.2. Melatonin Alleviates DSS-Induced Colitis in Obese Mice
2.3. Melatonin Attenuates DSS-Induced Inflammation and Apoptosis in Obese Mice
2.4. Melatonin Enhances Antioxidant Activities to Improve Obesity-Related Colitis
2.5. Melatonin Reverses Intestinal Barrier Integrity in DSS-Treated Obese Mice
2.6. Melatonin Inhibits Lipolysis and Fatty Acid Transport in the White Adipose Tissues of DSS-Treated Obese Mice
2.7. Melatonin Mediates Adipocyte-Induced Autophagy in DSS-Treated Obese Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Disease Activity Index (DAI)
4.3. Fecal Occult Blood Testing
4.4. Myeloperoxidase (MPO) Activity Assay
4.5. Histological Analysis
4.6. Biochemical Analysis
4.7. Antioxidant Activity Assay
4.8. MDA Activity Assay
4.9. Colony Forming Units Measurement
4.10. Gene Expression Analysis
4.11. Western Blot Analysis and Immunofluorescence
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, R.; Wankell, M.; Ahlenstiel, G.; Hebbard, L. The role of obesity in inflammatory bowel disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 63–72. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients with Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Surmiak, M.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Danielak, A.; Ptak-Belowska, A.; et al. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules 2019, 9, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Xu, P.; Xi, Y.; Zhu, J.; Zhang, M.; Luka, Z.; Stolz, D.B.; Cai, X.; Xie, Y.; Xu, M.; Ren, S.; et al. Intestinal Sulfation Is Essential to Protect Against Colitis and Colonic Carcinogenesis. Gastroenterology 2021, 161, 271–286. [Google Scholar] [CrossRef]
- Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef]
- Ehrlich, A.C.; Patel, S.; Meillier, A.; Rothstein, R.D.; Friedenberg, F.K. Chemoprevention of colorectal cancer in inflammatory bowel disease. Expert Rev. Anticancer Ther. 2017, 17, 247–255. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Pan, S.; Xu, P.; Xue, T.; Wang, J.; Guo, Y.; Jia, L.; Qiao, X.; Li, L.; Zhai, Y. Melatonin Orchestrates Lipid Homeostasis through the Hepatointestinal Circadian Clock and Microbiota during Constant Light Exposure. Cells 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyanarayanan, S.K.; Su, H.; Lin, Y.W.; Su, K.P. Circadian Rhythm and Melatonin in the Treatment of Depression. Curr. Pharm. Des. 2018, 24, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, A.S.; Bernikova, O.G.; Mikhaleva, N.J.; Khramova, D.S.; Ovechkin, A.O.; Demidova, M.M.; Platonov, P.G.; Azarov, J.E. Melatonin Prevents Early but Not Delayed Ventricular Fibrillation in the Experimental Porcine Model of Acute Ischemia. Int. J. Mol. Sci. 2020, 22, 328. [Google Scholar] [CrossRef]
- Ostrycharz, E.; Wasik, U.; Kempinska-Podhorodecka, A.; Banales, J.M.; Milkiewicz, P.; Milkiewicz, M. Melatonin Protects Cholangiocytes from Oxidative Stress-Induced Proapoptotic and Proinflammatory Stimuli via miR-132 and miR-34. Int. J. Mol. Sci. 2020, 21, 9667. [Google Scholar] [CrossRef]
- Aouichat, S.; Navarro-Alarcon, M.; Alarcon-Guijo, P.; Salagre, D.; Ncir, M.; Zourgui, L.; Agil, A. Melatonin Improves Endoplasmic Reticulum Stress-Mediated IRE1alpha Pathway in Zucker Diabetic Fatty Rat. Pharmaceuticals 2021, 14, 232. [Google Scholar] [CrossRef]
- Chao, Y.C.; Lee, K.Y.; Wu, S.M.; Kuo, D.Y.; Shueng, P.W.; Lin, C.W. Melatonin Downregulates PD-L1 Expression and Modulates Tumor Immunity in KRAS-Mutant Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 5649. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shen, M.; Chen, Y.; Wei, Y.; Tao, J.; Liu, H. Melatonin Represses Mitophagy to Protect Mouse Granulosa Cells from Oxidative Damage. Biomolecules 2021, 11, 968. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepanska, B.; Ostrowska, J.; Kozlowska, J.; Szota, Z.; Brozyna, A.A.; Dreier, R.; Reiter, R.J.; Slominski, A.T.; Steinbrink, K.; Kleszczynski, K. Evaluation of Polymeric Matrix Loaded with Melatonin for Wound Dressing. Int. J. Mol. Sci. 2021, 22, 5658. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, T.; Lee, T.H. Cellular Mechanisms of Melatonin: Insight from Neurodegenerative Diseases. Biomolecules 2020, 10, 1158. [Google Scholar] [CrossRef]
- Roy, J.; Tsui, K.C.; Ng, J.; Fung, M.L.; Lim, L.W. Regulation of Melatonin and Neurotransmission in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 6841. [Google Scholar] [CrossRef]
- Lalanne, S.; Fougerou-Leurent, C.; Anderson, G.M.; Schroder, C.M.; Nir, T.; Chokron, S.; Delorme, R.; Claustrat, B.; Bellissant, E.; Kermarrec, S.; et al. Melatonin: From Pharmacokinetics to Clinical Use in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 1490. [Google Scholar] [CrossRef] [PubMed]
- Mhalhel, K.; Germana, A.; Abbate, F.; Guerrera, M.C.; Levanti, M.; Laura, R.; Montalbano, G. The Effect of Orally Supplemented Melatonin on Larval Performance and Skeletal Deformities in Farmed Gilthead Seabream (Sparus aurata). Int. J. Mol. Sci. 2020, 21, 9597. [Google Scholar] [CrossRef]
- Singh, M.K.; Dias, B.K.M.; Garcia, C.R.S. Role of Melatonin in the Synchronization of Asexual Forms in the Parasite Plasmodium falciparum. Biomolecules 2020, 10, 1243. [Google Scholar] [CrossRef]
- Sotak, M.; Mrnka, L.; Pacha, J. Heterogeneous expression of melatonin receptor MT1 mRNA in the rat intestine under control and fasting conditions. J. Pineal Res. 2006, 41, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Localization of melatonin in the digestive tract of the rat. Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Horm. Res. 1980, 12, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Chamanara, M.; Rashidian, A.; Mehr, S.E.; Dehpour, A.R.; Shirkohi, R.; Akbarian, R.; Abdollahi, A.; Rezayat, S.M. Melatonin ameliorates TNBS-induced colitis in rats through the melatonin receptors: Involvement of TLR4/MyD88/NF-kappaB signalling pathway. Inflammopharmacology 2019, 27, 361–371. [Google Scholar] [CrossRef]
- Esteban-Zubero, E.; Lopez-Pingarron, L.; Alatorre-Jimenez, M.A.; Ochoa-Moneo, P.; Buisac-Ramon, C.; Rivas-Jimenez, M.; Castan-Ruiz, S.; Antonanzas-Lombarte, A.; Tan, D.X.; Garcia, J.J.; et al. Melatonin’s role as a co-adjuvant treatment in colonic diseases: A review. Life Sci. 2017, 170, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.W.; Wang, C.D. Melatonin alleviates circadian rhythm disruption exacerbating DSS-induced colitis by inhibiting the distribution of HMGB1 in intestinal tissues. Int. Immunopharmacol. 2019, 73, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, Z.; Keefer, L.; Farhadi, A.; Stepanski, E.; Sedghi, S.; Keshavarzian, A. Impact of sleep disturbances in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2007, 22, 1748–1753. [Google Scholar] [CrossRef]
- Ranjbaran, Z.; Keefer, L.; Stepanski, E.; Farhadi, A.; Keshavarzian, A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm. Res. 2007, 56, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Wisniewska-Jarosinska, M.; Walecka-Kapica, E.; Klupinska, G.; Jaworek, J.; Chojnacki, J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J. Physiol. Pharmacol. 2011, 62, 327–334. [Google Scholar] [PubMed]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020, 10, 2232. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of Melatonin on Intestinal Microbiota and Oxidative Stress in Colitis Mice. Biomed. Res. Int. 2018, 2018, 2607679. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzon, E.; Esposito, E.; Crisafulli, C.; Riccardi, L.; Muia, C.; Di Bella, P.; Meli, R.; Cuzzocrea, S. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J. Pineal Res. 2006, 41, 363–373. [Google Scholar] [CrossRef]
- Nduhirabandi, F.; Lamont, K.; Albertyn, Z.; Opie, L.H.; Lecour, S. Role of toll-like receptor 4 in melatonin-induced cardioprotection. J. Pineal Res. 2016, 60, 39–47. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blain, A.; Cattan, S.; Beaugerie, L.; Carbonnel, F.; Gendre, J.P.; Cosnes, J. Crohn’s disease clinical course and severity in obese patients. Clin. Nutr. 2002, 21, 51–57. [Google Scholar] [CrossRef]
- Losurdo, G.; La Fortezza, R.F.; Iannone, A.; Contaldo, A.; Barone, M.; Ierardi, E.; Di Leo, A.; Principi, M. Prevalence and associated factors of obesity in inflammatory bowel disease: A case-control study. World J. Gastroenterol. 2020, 26, 7528–7537. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, L.; Amati, L.; Magrone, T.; Pellegrino, N.M.; Jirillo, E.; Caccavo, D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: Biological and clinical significance. J. Endotoxin Res. 2000, 6, 205–214. [Google Scholar] [PubMed]
- Muzes, G.; Molnar, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugtveit, J.; Brandtzaeg, P.; Halstensen, T.S.; Fausa, O.; Scott, H. Increased macrophage subset in inflammatory bowel disease: Apparent recruitment from peripheral blood monocytes. Gut 1994, 35, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, M.; Koji, T.; Makiyama, K.; Kobayashi, N.; Nakane, P.K. Apoptosis of crypt epithelial cells in ulcerative colitis. J. Pathol. 1996, 180, 152–159. [Google Scholar] [CrossRef]
- Strater, J.; Wellisch, I.; Riedl, S.; Walczak, H.; Koretz, K.; Tandara, A.; Krammer, P.H.; Moller, P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: A possible role in ulcerative colitis. Gastroenterology 1997, 113, 160–167. [Google Scholar] [CrossRef]
- Seril, D.N.; Liao, J.; Yang, G.Y.; Yang, C.S. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibi, S.; de Sousa Moraes, L.F.; Lebow, N.; Zhu, M.J. Dietary Green Pea Protects against DSS-Induced Colitis in Mice Challenged with High-Fat Diet. Nutrients 2017, 9, 509. [Google Scholar] [CrossRef] [Green Version]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Magierowski, M.; Surmiak, M.; Kwiecien, S.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Brzozowski, T. Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers. Nutrients 2019, 11, 1127. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, X. Obesity Promotes Experimental Colitis by Increasing Oxidative Stress and Mitochondrial Dysfunction in the Colon. Inflammation 2020, 43, 1884–1892. [Google Scholar] [CrossRef]
- Akcan, A.; Kucuk, C.; Sozuer, E.; Esel, D.; Akyildiz, H.; Akgun, H.; Muhtaroglu, S.; Aritas, Y. Melatonin reduces bacterial translocation and apoptosis in trinitrobenzene sulphonic acid-induced colitis of rats. World J. Gastroenterol. 2008, 14, 918–924. [Google Scholar] [CrossRef]
- Rocha, R.; Santana, G.O.; Almeida, N.; Lyra, A.C. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br. J. Nutr. 2009, 101, 676–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, L.G.; Leonel, A.J.; Aguilar, E.C.; Batista, N.V.; Alves, A.C.; Coimbra, C.C.; Ferreira, A.V.; de Faria, A.M.; Cara, D.C.; Alvarez Leite, J.I. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Chamaillard, M.; Gonzalez, F.; Beclin, E.; Decourcelle, C.; Antunes, L.; Gay, J.; Neut, C.; Colombel, J.F.; Desreumaux, P. Mesenteric fat in Crohn’s disease: A pathogenetic hallmark or an innocent bystander? Gut 2007, 56, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, R.; White, E. Autophagy, stress, and cancer metabolism: What doesn’t kill you makes you stronger. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 389–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Jin, H.; Qiang, Y.; Wu, S.; Yan, C.; Han, M.; Xiao, T.; Yan, N.; An, H.; Zhou, X.; et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int. Immunopharmacol. 2016, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Fierce, Y.; Treuting, P.M.; Brabb, T.; Maggio-Price, L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J. Nutr. 2013, 143, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Logt, E.M.; Blokzijl, T.; van der Meer, R.; Faber, K.N.; Dijkstra, G. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme. J. Nutr. Biochem. 2013, 24, 1159–1165. [Google Scholar] [CrossRef]
- Perez, C.C.; Tobar, I.D.; Jimenez, E.; Castaneda, D.; Rivero, M.B.; Concepcion, J.L.; Chiurillo, M.A.; Bonfante-Cabarcas, R. Kinetic and molecular evidences that human cardiac muscle express non-M2 muscarinic receptor subtypes that are able to interact themselves. Pharmacol. Res. 2006, 54, 345–355. [Google Scholar] [CrossRef]
- Mahler, M.; Bristol, I.J.; Leiter, E.H.; Workman, A.E.; Birkenmeier, E.H.; Elson, C.O.; Sundberg, J.P. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am. J. Physiol. 1998, 274, G544–G551. [Google Scholar] [CrossRef] [Green Version]
- Barollo, M.; Medici, V.; D’Inca, R.; Banerjee, A.; Ingravallo, G.; Scarpa, M.; Patak, S.; Ruffolo, C.; Cardin, R.; Sturniolo, G.C. Antioxidative potential of a combined therapy of anti TNFalpha and Zn acetate in experimental colitis. World J. Gastroenterol. 2011, 17, 4099–4103. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Nunez, M.L.; Gonzalez de Mejia, E.; Loarca-Pina, G. Moringa oleifera leaves alleviated inflammation through downregulation of IL-2, IL-6, and TNF-alpha in a colitis-associated colorectal cancer model. Food Res. Int. 2021, 144, 110318. [Google Scholar] [CrossRef] [PubMed]
- Luzardo-Ocampo, I.; Campos-Vega, R.; Gonzalez de Mejia, E.; Loarca-Pina, G. Consumption of a baked corn and bean snack reduced chronic colitis inflammation in CD-1 mice via downregulation of IL-1 receptor, TLR, and TNF-alpha associated pathways. Food Res. Int. 2020, 132, 109097. [Google Scholar] [CrossRef] [PubMed]
- Schicho, R.; Bashashati, M.; Bawa, M.; McHugh, D.; Saur, D.; Hu, H.M.; Zimmer, A.; Lutz, B.; Mackie, K.; Bradshaw, H.B.; et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm. Bowel Dis. 2011, 17, 1651–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwanke, R.C.; Marcon, R.; Meotti, F.C.; Bento, A.F.; Dutra, R.C.; Pizzollatti, M.G.; Calixto, J.B. Oral administration of the flavonoid myricitrin prevents dextran sulfate sodium-induced experimental colitis in mice through modulation of PI3K/Akt signaling pathway. Mol. Nutr. Food Res. 2013, 57, 1938–1949. [Google Scholar] [CrossRef]
- Silvestri, C.; Pagano, E.; Lacroix, S.; Venneri, T.; Cristiano, C.; Calignano, A.; Parisi, O.A.; Izzo, A.A.; Di Marzo, V.; Borrelli, F. Fish Oil, Cannabidiol and the Gut Microbiota: An Investigation in a Murine Model of Colitis. Front. Pharmacol. 2020, 11, 585096. [Google Scholar] [CrossRef]
- Borniquel, S.; Jadert, C.; Lundberg, J.O. Dietary conjugated linoleic acid activates PPARgamma and the intestinal trefoil factor in SW480 cells and mice with dextran sulfate sodium-induced colitis. J. Nutr. 2012, 142, 2135–2140. [Google Scholar] [CrossRef] [Green Version]
- Salim, S.Y.; Soderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Lih-Brody, L.; Powell, S.R.; Collier, K.P.; Reddy, G.M.; Cerchia, R.; Kahn, E.; Weissman, G.S.; Katz, S.; Floyd, R.A.; McKinley, M.J.; et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996, 41, 2078–2086. [Google Scholar] [CrossRef]
- Buffinton, G.D.; Doe, W.F. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic. Biol. Med. 1995, 19, 911–918. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Bulbuloglu, E.; Kantarceken, B.; Ciralik, H.; Kurutas, E.B.; Buyukbese, M.A.; Gumusalan, Y. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig. Dis. Sci. 2006, 51, 488–494. [Google Scholar] [CrossRef]
- Koch, T.R.; Yuan, L.X.; Stryker, S.J.; Ratliff, P.; Telford, G.L.; Opara, E.C. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig. Dis. Sci. 2000, 45, 1814–1819. [Google Scholar] [CrossRef]
- Mahdavi, N.S.; Talebi, A.; Minaiyan, M. Ameliorative effect of galantamine on acetic acid-induced colitis in rats. Res. Pharm. Sci. 2019, 14, 391–399. [Google Scholar]
- Crespo, I.; San-Miguel, B.; Prause, C.; Marroni, N.; Cuevas, M.J.; Gonzalez-Gallego, J.; Tunon, M.J. Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS ONE 2012, 7, e50407. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, D.; Li, M.; Wang, H.; He, Q.; Wang, Y.; Yu, H.; Tian, D.; Yu, Q. SLC26A3 (DRA) prevents TNF-alpha-induced barrier dysfunction and dextran sulfate sodium-induced acute colitis. Lab. Investig. 2018, 98, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagala-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Wild, G.E.; Drozdowski, L.; Tartaglia, C.; Clandinin, M.T.; Thomson, A.B. Nutritional modulation of the inflammatory response in inflammatory bowel disease--from the molecular to the integrative to the clinical. World J. Gastroenterol. 2007, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Braun, K.; Oeckl, J.; Westermeier, J.; Li, Y.; Klingenspor, M. Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J. Exp. Biol. 2018, 221, jeb165381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Aranda, A.; Fernandez-Vazquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.X.; Reiter, R.J.; Agil, A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef]
- Liu, K.; Yu, W.; Wei, W.; Zhang, X.; Tian, Y.; Sherif, M.; Liu, X.; Dong, C.; Wu, W.; Zhang, L.; et al. Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 2019, 60, 767–782. [Google Scholar] [CrossRef]
- Ryu, V.; Zarebidaki, E.; Albers, H.E.; Xue, B.; Bartness, T.J. Short photoperiod reverses obesity in Siberian hamsters via sympathetically induced lipolysis and Browning in adipose tissue. Physiol. Behav. 2018, 190, 11–20. [Google Scholar] [CrossRef]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, I.; Shim, H.M.; Cho, H. Melatonin ameliorates SGLT2 inhibitor-induced diabetic ketoacidosis by inhibiting lipolysis and hepatic ketogenesis in type 2 diabetic mice. J. Pineal Res. 2020, 68, e12623. [Google Scholar] [CrossRef]
- Truter, D.; Chellan, N.; Strijdom, H.; Webster, I.; Rawstorne, J.; Kotze, S.H. Histomorphological changes in the pancreas and kidney and histopathological changes in the liver in male Wistar rats on antiretroviral therapy and melatonin treatment. Acta Histochem. 2018, 120, 347–355. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaldoun, S.A.; Emond-Boisjoly, M.A.; Chateau, D.; Carriere, V.; Lacasa, M.; Rousset, M.; Demignot, S.; Morel, E. Autophagosomes contribute to intracellular lipid distribution in enterocytes. Mol. Biol. Cell 2014, 25, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Czaja, M.J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013, 20, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef]
- Lin, N.Y.; Stefanica, A.; Distler, J.H. Autophagy: A key pathway of TNF-induced inflammatory bone loss. Autophagy 2013, 9, 1253–1255. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.N.; Viarengo, A.; Donkin, P.; Hawkins, A.J. Autophagic and lysosomal reactions to stress in the hepatopancreas of blue mussels. Aquat. Toxicol. 2007, 84, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2019, 38, e101812. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Hsiao, T.Y.; Chien, C.T.; Lai, M.K. Ischemic conditioning by short periods of reperfusion attenuates renal ischemia/reperfusion induced apoptosis and autophagy in the rat. J. Biomed. Sci. 2009, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Zeng, M.; Wei, X.; Wu, Z.; Li, W.; Li, B.; Zhen, Y.; Chen, J.; Wang, P.; Fei, Y. NF-kappaB-mediated induction of autophagy in cardiac ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2013, 436, 180–185. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Lv, W.J.; Liu, C.; Yu, L.Z.; Zhou, J.H.; Li, Y.; Xiong, Y.; Guo, A.; Chao, L.M.; Qu, Q.; Wei, G.W.; et al. Melatonin Alleviates Neuroinflammation and Metabolic Disorder in DSS-Induced Depression Rats. Oxidat. Med. Cell. Longev. 2020, 2020, 1241894. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Ren, W.; Wang, P.; Yan, J.; Liu, G.; Zeng, B.; Hussain, T.; Peng, C.; Yin, J.; Li, T.; Wei, H.; et al. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J. Pineal Res. 2018, 64, e12448. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, S.; Liu, Z.; Wei, H.; Zhang, L.; He, M.; Pei, F.; Zhang, J.; Sun, Q.; Duan, L. Increased Expression of Colonic Mucosal Melatonin in Patients with Irritable Bowel Syndrome Correlated with Gut Dysbiosis. Genom. Proteom. Bioinform. 2020, 18, 708–720. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Xu, P.; Hong, F.; Wang, J.; Cong, Y.; Dai, S.; Wang, S.; Wang, J.; Jin, X.; Wang, F.; Liu, J.; et al. Microbiome Remodeling via the Montmorillonite Adsorption-Excretion Axis Prevents Obesity-related Metabolic Disorders. EBioMedicine 2017, 16, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Hong, F.; Wang, J.; Wang, J.; Zhao, X.; Wang, S.; Xue, T.; Xu, J.; Zheng, X.; Zhai, Y. DBZ is a putative PPARgamma agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2690–2701. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Hong, F.; Li, L.; Guo, Y.; Qiao, X.; Zhang, J.; Xu, P.; Zhai, Y. Melatonin Attenuates Dextran Sodium Sulfate Induced Colitis in Obese Mice. Pharmaceuticals 2021, 14, 822. https://doi.org/10.3390/ph14080822
Pan S, Hong F, Li L, Guo Y, Qiao X, Zhang J, Xu P, Zhai Y. Melatonin Attenuates Dextran Sodium Sulfate Induced Colitis in Obese Mice. Pharmaceuticals. 2021; 14(8):822. https://doi.org/10.3390/ph14080822
Chicago/Turabian StylePan, Shijia, Fan Hong, Letong Li, Yuan Guo, Xiaoxiao Qiao, Jia Zhang, Pengfei Xu, and Yonggong Zhai. 2021. "Melatonin Attenuates Dextran Sodium Sulfate Induced Colitis in Obese Mice" Pharmaceuticals 14, no. 8: 822. https://doi.org/10.3390/ph14080822
APA StylePan, S., Hong, F., Li, L., Guo, Y., Qiao, X., Zhang, J., Xu, P., & Zhai, Y. (2021). Melatonin Attenuates Dextran Sodium Sulfate Induced Colitis in Obese Mice. Pharmaceuticals, 14(8), 822. https://doi.org/10.3390/ph14080822