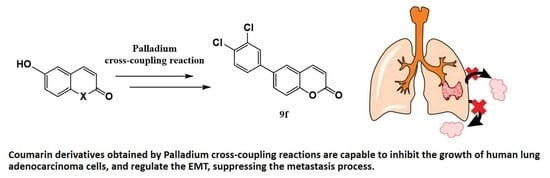
Coumarin Derivatives Exert Anti-Lung Cancer Activity by Inhibition of Epithelial–Mesenchymal Transition and Migration in A549 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Effects of Coumarin Derivatives on Cell Viability
2.2.2. Effect of 9f on IL-1β-Induced EMT in A549 Cells
3. Materials and Methods
3.1. Compounds (Synthetic Coumarins)
3.2. Biological Assays
3.2.1. Cell line and Cell Culture
3.2.2. Cell Viability Assay and Treatment
3.2.3. Epithelial-to-Mesenchymal Transition (EMT) Induction and Coumarin Derivatives Treatment
3.2.4. Immunofluorescence Staining
3.2.5. In Vitro Scratch Wound Healing Assay
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafar, S.N.; Siddiqui, A.H.; Channa, R.; Ahmed, S.; Javed, A.A.; Bafford, A. Estimating the Global Demand and Delivery of Cancer Surgery. World J. Surg. 2019, 43, 2203–2210. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torres, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 24–44. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Pearson, G.W. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J. Clin. Med. 2019, 8, 646. [Google Scholar] [CrossRef] [Green Version]
- Marcucci, F.; Stassi, G.; De Maria, R. Epithelial-mesenchymal transition: A new target in anticancer drug discovery. Nat. Rev. Drug Discov. 2016, 15, 311–325. [Google Scholar] [CrossRef]
- Cho, E.S.; Kang, H.E.; Kim, N.H.; Yook, J.I. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch. Pharm. Res. 2019, 42, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.S.A.; Mendonça, F.J.B., Jr. Coumarins: Synthetic Approaches and Pharmacological Importance. In Natural Products and Drug Discovery: From Pharmacochemistry to Pharmacological Approaches, 1st ed.; Diniz, M.F.F.M., Scotti, L., Scotti, M.T., Alves, M.F., Eds.; Editora UFPB: João Pessoa, Brazil, 2018; pp. 245–274. [Google Scholar]
- Zhang, S.-G.; Liang, C.-G.; Sun, Y.-Q.; Teng, P.; Wang, J.-Q.; Zhang, W.-H. Design, synthesis and antifungal activities of novel pyrrole- and pyrazole-substituted coumarin derivatives. Mol. Divers. 2019, 23, 915–925. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Jalalifard, Z.; Fathanbari, G.P. Green synthesis of bis-coumarin derivatives using Fe(SD)3 as a catalyst and investigation of their biological activities. J. Chin. Chem. Soc. 2020, 67, 172–182. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Ibraheem, H.H.; Jassim, L.S.; Al-Amiery, A.A. Antioxidant activity of coumarin compounds. ANJS 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Wang, T.; Peng, T.; Wen, X.; Wang, G.; Liu, S.; Sun, Y.; Zhang, S.; Wang, L. Design, synthesis and evaluation of 3-substituted coumarin derivatives as anti-inflammatory agents. Chem. Pharm. Bull. 2020, c19-01085. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, T.K.; Batran, R.Z.; Elseginy, S.A.; Ali, M.M.; Mahmoud, A.E. Synthesis, anticancer effect and molecular modeling of new thiazolylpyrazolyl coumarin derivatives targeting VEGFR-2 kinase and inducing cell cycle arrest and apoptosis. Bioorg. Chem. 2019, 85, 253–273. [Google Scholar] [CrossRef]
- Kasperkiewicz, K.; Ponczek, M.B.; Owczarek, J.; Guga, P.; Budzisz, E. Antagonists of vitamin K—Popular coumarin drugs and new synthetic and natural coumarin derivatives. Molecules 2020, 25, 1465. [Google Scholar] [CrossRef] [Green Version]
- Thornes, R.D.; Daly, L.; Lynch, G.; Breslin, B.; Browne, H.; Browne, H.Y.; Corrigan, T.; Daly, P.; Edwards, G.; Gaffney, E.; et al. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J. Cancer. Res. Clin. Oncol. 1994, 120, S32–S34. [Google Scholar] [CrossRef]
- Marshall, M.E.; Mohler, J.L.; Edmonds, K.; Williams, B.; Butler, K.; Ryles, M.; Weiss, L.; Urban, D.; Bueschen, A.; Markiewicz, M.; et al. An updated review of the clinical development of coumarin (1,2-benzopyrone) and 7-hydroxycoumarin. J. Cancer Res. Clin. Oncol. 1994, 120, S39–S42. [Google Scholar] [CrossRef]
- von Angerer, E.; Kager, M.; Maucher, A. Anti-tumour activity of coumarin in prostate and mammary cancer models. J. Cancer Res. Clin. Oncol. 1994, 120, S14–S16. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, J.S.; Prado-Garcia, H.; Aguilar-Cazares, D.; Molina-Guarneros, J.A.; Morales-Fuentes, J.; Mandoki, J.J. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer. 2004, 43, 275–283. [Google Scholar] [CrossRef]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef]
- Dandriyal, J.; Singla, R.; Kumar, M.; Jaitak, V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016, 119, 141–168. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Z. Coumarin-containing hybrids and their anticancer activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Klenkar, J.; Molnar, M. Natural and synthetic coumarins as potential anticancer agents. J. Chem. Pharm. Res. 2015, 7, 1223–1238. [Google Scholar]
- Kawaii, S.; Tomono, Y.; Ogawa, K.; Sugiura, M.; Yano, M.; Yoshizawa, Y. The anti-proliferative effect of coumarins on several cancer cell lines. Anticancer Res. 2001, 21, 917–923. [Google Scholar]
- Kumar, M.; Singla, R.; Dandriyal, J.; Jaitak, V. Coumarin Derivatives as Anticancer Agents for Lung Cancer Therapy: A Review. Anti-Cancer Agents Med. Chem. 2018, 8, 964–984. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.G.; Yuan, Y.L. Synthesis and evaluation of coumarin derivatives against human lung cancer cell lines. Braz. J. Med. Biol. Res. 2017, 50, e6455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, C.F.; Pan, L.M.; Gao, Z.L. 7,8-Dihydroxycoumarin inhibits A549 human lung adenocarcinoma cell proliferation by inducing apoptosis via suppression of Akt/NF-κB signaling. Exp. Ther. Med. 2013, 5, 1770–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, M.A.; Joseph, M.Y.; Latinwo, L.M.; Badisa, V.; Cooperwood, J.S. In vitro evaluation of 3-arylcoumarin derivatives in A549 cell line. Anticancer Res. 2015, 35, 653–659. [Google Scholar] [PubMed]
- Musa, M.A.; Badisa, L.D.V.; Latinwo, L.M.; Patterson, T.A.; Owens, A.M. Coumarin-based Benzopyranone Derivatives Induced Apoptosis in Human Lung (A549) Cancer Cells. Anticancer Res. 2012, 32, 4271–4276. [Google Scholar]
- Khaghanzadeh, N.; Mojtahedi, Z.; Ramezani, M.; Erfani, N.; Ghaderi, A. Umbelliprenin is cytotoxic against QU-DB large cell lung cancer cell line but anti-proliferative against A549 adenocarcinoma cells. DARU J. Pharm. Sci. 2012, 20, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Xiaoman, X.; Zhang, Y.; Qu, D.; Jiang, T.; Li, S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30, 33. [Google Scholar]
- Basanagouda, M.; Jambagi, V.B.; Barigidad, N.N.; Laxmeshwar, S.S.; Devaru, V. Narayanachar. Synthesis, structure-activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and anti-mycobacterial agents. Eur. J. Med. Chem. 2014, 74, 225–233. [Google Scholar] [CrossRef]
- Belluti, F.; Fontana, G.; Dal Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.R.; Liu, H.S.; Cheng, M.; Xia, P.; Qian, K.; Wu, P.C.; Lai, C.Y.; Xia, Y.; Yang, Z.Y.; et al. Antitumor agents 292. Design, synthesis and pharmacological study of S- and O-substituted 7-mercapto- or hydroxy-coumarins and chromones as potent cytotoxic agents. Eur. J. Med. Chem. 2012, 49, 74–85. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. Engl. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Mori, A.; Ahmed, M.S.M.; Sekiguchi, A.; Masui, K.; Koike, T. Sonogashira coupling with aqueous ammonia. Chem. Lett. 2002, 31, 756–757. [Google Scholar] [CrossRef]
- Bellina, F.; Carpita, A.; Rossi, R. Palladium catalysts for the Suzuki cross-coupling reaction: An overview of recent advances. Synthesis 2004, 2004, 2419–2440. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Hu, Q.-S. Room temperature nickel(0)-catalyzed suzuki-miyaura cross-couplings of activated alkenyl tosylates: Efficient synthesis of 4-substituted coumarins and 4-substituted 2-(5H)-furanones. Adv. Synth. Catal. 2004, 346, 1635–1637. [Google Scholar] [CrossRef]
- Završnik, D.; Muratović, S.; Makuc, D.; Plavec, J.; Cetina, M.; Nagl, A.; De Clercq, E.; Balzarini, J.; Mintas, M. Benzylidene-bis-(4-hydroxycoumarin) and benzopyrano-coumarin derivatives: Synthesis, 1H/13C-NMR conformational and X-ray crystal structure studies and in vitro antiviral activity evaluations. Molecules 2011, 16, 6023–6040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadafora, M.; Postupalenko, V.Y.; Shvadchak, V.V.; Klymchenko, A.S.; Mély, Y.; Burger, A.; Benhida, R. Efficient synthesis of ratiometric fluorescent nucleosides featuring 3-hydroxychromone nucleobases. Tetrahedron 2009, 65, 7809–7816. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nishizono, N.; Kobayashi, D.; Yoshimura, T.; Wada, K.; Oda, K. Evaluation of synthesized coumarin derivatives on aromatase inhibitory activity. Bioorg. Med. Chem. Lett. 2017, 27, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Chorley, D.F.; Furkert, D.P.; Brimble, M.A. Synthesis of the spiroketal core of the pinnatifinoside family of natural products. Eur. J. Org. Chem. 2016, 2016, 314–319. [Google Scholar] [CrossRef]
- Manolikakes, G.; Dong, Z.; Mayr, H.; Li, J.; Knochel, P. Negishi Cross-Coupling Compatible with Unprotected Amide Functions. Chem. Eur. J. 2009, 15, 1324–1328. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017, 12, 833–842. [Google Scholar] [CrossRef]
- Gavert, N.; Ben-Ze´ev, A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol. Med. 2008, 14, 199–209. [Google Scholar] [CrossRef]
- Bruzzese, F.; Leone, A.; Rocco, M.; Carbone, C.; Piro, G.; Caraglia, M.; Di Gennaro, E.; Budillon, A. HDAC inhibitor vorinostat enhances the antitumor effect of gefitinib in squamous cell carcinoma of head and neck by modulating ErbB receptor expression and reverting EMT. J. Cell Physiol. 2011, 226, 2378–2390. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, A.M. Epithelial mesenchymal interactions in cancer and development. Cell 2001, 105, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Yu, H.H.; Liu, Y.S.; Wang, Y.S.; Zhao, W.H. Esculetin enhances the inhibitory effect of 5-Fluorouracil on the proliferation, migration and epithelial-mesenchymal transition of colorectal cancer. Cancer Biomark. 2019, 24, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.N.; Ni, X.Y.; Yan, H.Q.; Shi, L.; Lu, N.N.; Wang, Y.N.; Li, Q.; Gao, F.G. Interleukin 6-triggered ataxia-telangiectasia mutated kinase activation facilitates epithelial-to-mesenchymal transition in lung cancer by upregulating vimentin expression. Exp. Cell Res. 2019, 381, 165–171. [Google Scholar] [CrossRef]
- Wang, T.-C.; Chen, Y.-L.; Tzeng, C.-C.; Liou, S.-S.; Tzeng, W.-F.; Chang, Y.-L.; Teng, C.-M. α-Methylidene-γ-butyrolactones: Synthesis and evaluation of quinolin-2(1H)-one derivatives. Helv. Chim. Acta 1998, 81, 1038–1047. [Google Scholar] [CrossRef]
- Plougastel, L.; Pattanayak, M.R.; Riomet, M.; Bregant, S.; Sallustrau, A.; Nothisen, M.; Wagner, A.; Audisio, D.; Taran, F. Sydnone-based turn-on fluorogenic probes for no-wash protein labeling and in-cell imaging. Chem. Commun. 2019, 55, 4582–4585. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, M.L.N. Pot-economic synthesis of diarylpyrazoles and pyrimidines involving Pd-catalyzed cross-coupling of 3-trifloxychromone and triarylbismuth. J. Chem. Sci. 2018, 130, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Starčević, Š.; Brožič, P.; Turk, S.; Cesar, J.; Rižner, T.L.; Gobec, S. Synthesis and biological evaluation of (6- and 7-phenyl) coumarin derivatives as selective nonsteroidal inhibitors of 17β-hydroxysteroid dehydrogenase type 1. J. Med. Chem. 2011, 54, 248–261. [Google Scholar] [CrossRef]
- Das, S.G.; Srinivasan, B.; Hermanson, D.L.; Bleeker, N.P.; Doshi, J.M.; Tang, R.; Beck, W.T.; Xing, C. Structure-activity relationship and molecular mechanisms of ethyl 2-amino-6-(3,5-dimethoxyphenyl)-4-(2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (CXL017) and its analogues. J. Med. Chem. 2011, 54, 5937–5948. [Google Scholar] [CrossRef]
- Aridoss, G.; Zhou, B.; Hermanson, D.L.; Bleeker, N.P.; Xing, C. Structure-activity relationship (SAR) study of ethyl 2-amino-6-(3,5-dimethoxyphenyl)-4-(2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylated (CXL017) and the potential of the lead against multidrug resistance in cancer treatment. J. Med. Chem. 2012, 55, 5566–5581. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, J.; Peng, C.; Dai, N.; Tang, Z.; Jiao, Y.; Chen, J.; Xu, X. Synthesis of Unsymmetrical Aromatic Acetylenes by Diphenyl Chlorophosphate-Promoted Condensation Reaction of Aromatic Aldehydes and Sulfones. Chin. J. Org. Chem. 2017, 37, 3013–3018. [Google Scholar] [CrossRef]
- Elangovan, A.; Lin, J.-H.; Yang, S.-W.; Hsu, H.-Y.; Ho, T.-I. Synthesis and electrogenerated chemiluminescence of donor-substituted phenylethylcoumarins. J. Org. Chem. 2004, 69, 8086–8092. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.; Maka, V.K.; Payra, S.; Moorthy, J.N. Multifunctional porous organic polymers (POPs): Inverse adsorption of hydrogen over nitrogen, stabilization of Pd(0) nanoparticles, and catalytic cross-coupling reactions and reductions. J. Catal. 2020, 284, 61–71. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemoth. Rep. 1972, 3, 17–27. [Google Scholar]
- Wang, J.; Bao, L.; Yu, B.; Liu, Z.; Han, W.; Deng, C.; Guo, C. Interleukin-1β Promotes Epithelial-Derived Alveolar Elastogenesis via αvβ6 Integrin-Dependent TGF-β Activation. Cell Physiol. Biochem. 2015, 36, 2198–2216. [Google Scholar] [CrossRef]
- Cardoso, S.H.; de Oliveira, C.R.; Guimarães, A.S.; Nascimento, J.; de Oliveira Dos Santos Carmo, J.; de Souza Ferro, J.N.; de Carvalho Correia, A.C.; Barreto, E. Synthesis of newly functionalized 1,4-naphthoquinone derivatives and their effects on wound healing in alloxan-induced diabetic mice. Chem. Biol. Interact. 2018, 291, 55–64. [Google Scholar] [CrossRef]







| Cpd | X | R | Base | Catalyst. (5.0 mol %) | Solvent | Yield (%) |
|---|---|---|---|---|---|---|
| 8a | O | 7-(4-OCH3)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 71 |
| 8b | O | 7-(2-OCH3)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 84 |
| 8c | O | 7-(2-Cl)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 71 |
| 8d | O | 7-(Pyridin-4-yl) | K3PO4 | Pd(PPh3)4 | Toluene/EtOH/H2O (4:1:1) | 74 |
| 8e | O | 7-(4-CF3)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 78 |
| 8f | O | 7-(3,4-Cl)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 59 |
| 9a | O | 6-(4-OCH3)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 73 |
| 9b | O | 6-(3-OCH3)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 71 |
| 9c | O | 6-(2-OCH3)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 76 |
| 9d | O | 6-(4-Cl)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 45 |
| 9e | O | 6-(2-Cl)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 71 |
| 9f | O | 6-(3,4-Cl)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 68 |
| 9g | O | 6-(Pyridin-4-yl) | K3PO4 | Pd(PPh3)4 | Toluene/EtOH/H2O (4:1:1) | 82 |
| 7 | NCH3 | 6-(4-OCH3)-Ph | NaHCO3 | Pd(PPh3)4 | MeOH | 81 |
| 10 | - | 3-(4-Ome)-Ph | KF | Pd(Oac)2 | MeOH | 50 |
| Cpd | X | R | Base | Ligand/Catalyst | Additive | Yield % |
|---|---|---|---|---|---|---|
| 12a | O | Ph | Et3N | Pd(PPh3)2Cl2 | CuI | 75 |
| 12b | O | CH2OCH2Ph | Et3N | Pd(PPh3)2Cl2 | CuI | 38 |
| 12c | O | CH2OH | K2CO3 | S-Phos/Pd(Oac)2 | TBAI | 75 |
| 13a | O | Ph | K2CO3 | S-Phos/Pd(Oac)2 | TBAI | 78 |
| 13b | O | (CH2)3Ph | K2CO3 | S-Phos/Pd(Oac)2 | TBAI | 76 |
| 13c | O | CH2OH | K2CO3 | S-Phos/Pd(Oac)2 | TBAI | 72 |
| 11 | NCH3 | Ph | K2CO3 | S-Phos/Pd(Oac)2 | TBAI | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, R.S.A.; Carmo, J.d.O.d.S.; de Omena Silva, S.L.; Costa da Silva, C.R.A.; Souza, T.P.M.; Mélo, N.B.d.; Bourguignon, J.-J.; Schmitt, M.; Aquino, T.M.d.; Rodarte, R.S.; et al. Coumarin Derivatives Exert Anti-Lung Cancer Activity by Inhibition of Epithelial–Mesenchymal Transition and Migration in A549 Cells. Pharmaceuticals 2022, 15, 104. https://doi.org/10.3390/ph15010104
de Araújo RSA, Carmo JdOdS, de Omena Silva SL, Costa da Silva CRA, Souza TPM, Mélo NBd, Bourguignon J-J, Schmitt M, Aquino TMd, Rodarte RS, et al. Coumarin Derivatives Exert Anti-Lung Cancer Activity by Inhibition of Epithelial–Mesenchymal Transition and Migration in A549 Cells. Pharmaceuticals. 2022; 15(1):104. https://doi.org/10.3390/ph15010104
Chicago/Turabian Stylede Araújo, Rodrigo Santos Aquino, Julianderson de Oliveira dos Santos Carmo, Simone Lara de Omena Silva, Camila Radelley Azevedo Costa da Silva, Tayhana Priscila Medeiros Souza, Natália Barbosa de Mélo, Jean-Jacques Bourguignon, Martine Schmitt, Thiago Mendonça de Aquino, Renato Santos Rodarte, and et al. 2022. "Coumarin Derivatives Exert Anti-Lung Cancer Activity by Inhibition of Epithelial–Mesenchymal Transition and Migration in A549 Cells" Pharmaceuticals 15, no. 1: 104. https://doi.org/10.3390/ph15010104
APA Stylede Araújo, R. S. A., Carmo, J. d. O. d. S., de Omena Silva, S. L., Costa da Silva, C. R. A., Souza, T. P. M., Mélo, N. B. d., Bourguignon, J. -J., Schmitt, M., Aquino, T. M. d., Rodarte, R. S., Moura, R. O. d., Barbosa Filho, J. M., Barreto, E., & Mendonça-Junior, F. J. B. (2022). Coumarin Derivatives Exert Anti-Lung Cancer Activity by Inhibition of Epithelial–Mesenchymal Transition and Migration in A549 Cells. Pharmaceuticals, 15(1), 104. https://doi.org/10.3390/ph15010104