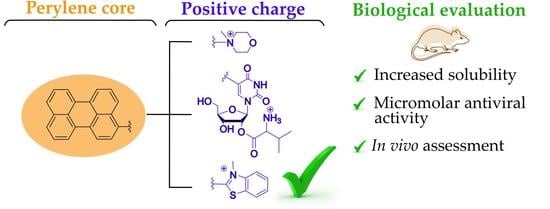
Cationic Perylene Antivirals with Aqueous Solubility for Studies In Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro Activity, Aqueous Solubility, and Serum Stability Assessment
2.3. In Vivo Studies
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for CuAAC Reaction
4-(2-(4-(Perylen-3-yl)-1H-1,2,3-triazol-1-yl)ethyl)morpholine 2a
4-(3-(4-(Perylen-3-yl)-1H-1,2,3-triazol-1-yl)propyl)morpholine 2b
3.2.2. General Procedure for Methylation with Methyl Iodide
4-Methyl-4-(2-(4-(perylen-3-yl)-1H-1,2,3-triazol-1-yl)ethyl)morpholin-4-ium iodide 3a
4-Methyl-4-(3-(4-(Perylen-3-yl)-1H-1,2,3-triazol-1-yl)propyl)morpholin-4-ium iodide 3b
3.2.3. 3′,5′-O-(Tetraisopropyldisiloxan-1,3-diyl)-5-(perylen-3-ylethynyl)uridine 5
3.2.4. 2′-O-(N-Boc-l-valinyl)-3′,5′-O-(tetraisopropyldisiloxane-1,3-diyl)-5-(perylen-3-ylethynyl)uridine 6
3.2.5. 2′-O-(N-Boc-l-valinyl)-5-(perylen-3-ylethynyl)uridine 7
3.2.6. 2′-O-l-Valinyl-5-(perylen-3-ylethynyl)uridine 8
3.2.7. 3-Methyl-2-(2-(perylen-3-yl)vinyl)benzo[d]thiazol-3-ium chloride 10
3.3. Solubility
3.4. In Vitro Biological Studies
3.4.1. Cells and Viruses
3.4.2. Methods
Cell viability Assay in Vero Cells
Cell Viability Assay in MDCK Cells
IAV Virus Yield Reduction Assay
SARS-CoV-2 and CHIKV Cytopathic Effect (CPE) Inhibition Test
Plasma Stability Test
3.5. In Vivo Biological Studies
3.5.1. Animals
3.5.2. Virus
3.5.3. Design of the Experiment to Determine the Antiviral Activity of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.F.W.; Chan, K.-H.; Kao, R.Y.T.; To, K.K.W.; Zheng, B.-J.; Li, C.P.Y.; Li, P.T.W.; Dai, J.; Mok, F.K.Y.; Chen, H.; et al. Broad-Spectrum Antivirals for the Emerging Middle East Respiratory Syndrome Coronavirus. J. Infect. 2013, 67, 606–616. [Google Scholar] [CrossRef]
- Raveh, A.; Delekta, P.C.; Dobry, C.J.; Peng, W.; Schultz, P.J.; Blakely, P.K.; Tai, A.W.; Matainaho, T.; Irani, D.N.; Sherman, D.H.; et al. Discovery of Potent Broad Spectrum Antivirals Derived from Marine Actinobacteria. PLoS ONE 2013, 8, e82318. [Google Scholar] [CrossRef] [PubMed]
- Carossino, M.; Thiry, E.; de la Grandière, A.; Barrandeguy, M.E. Novel Vaccination Approaches against Equine Alphavirus Encephalitides. Vaccine 2014, 32, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-D.; Meng, W.; Wang, X.-J.; Wang, H.-C.R. Broad-Spectrum Antiviral Agents. Front. Microbiol. 2015, 6, 517. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.; Aliota, M.; Bonnac, L. Broad-Spectrum Antiviral Strategies and Nucleoside Analogues. Viruses 2021, 13, 667. [Google Scholar] [CrossRef]
- Balazs, A.B.; Chen, J.; Hong, C.M.; Rao, D.S.; Yang, L.; Baltimore, D. Antibody-Based Protection against HIV Infection by Vectored Immunoprophylaxis. Nature 2012, 481, 81–84. [Google Scholar] [CrossRef]
- Johnson, P.R.; Schnepp, B.C.; Zhang, J.; Connell, M.J.; Greene, S.M.; Yuste, E.; Desrosiers, R.C.; Reed Clark, K. Vector-Mediated Gene Transfer Engenders Long-Lived Neutralizing Activity and Protection against SIV Infection in Monkeys. Nat. Med. 2009, 15, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, M.; Aschenbrenner, L.M.; Jensen, K.; Larson, J.L.; Fang, F. Sialidase-based Anti–Influenza Virus Therapy Protects against Secondary Pneumococcal Infection. J. Infect. Dis. 2010, 201, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Fan, J.; Haddad, C.S.; Ratia, K.; Broder, C.C.; Caffrey, M.; Prabhakar, B.S. Identification of a Broad-Spectrum Antiviral Small Molecule against Severe Acute Respiratory Syndrome Coronavirus and Ebola, Hendra, and Nipah Viruses by Using a Novel High-Throughput Screening Assay. J. Virol. 2014, 88, 4353–4365. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.M.; Kim, P.S. Mechanisms of Viral Membrane Fusion and Its Inhibition. Annu. Rev. Biochem. 2001, 70, 777–810. [Google Scholar] [CrossRef] [Green Version]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against Filovirus Diseases by a Novel Broad-Spectrum Nucleoside Analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef]
- Oestereich, L.; Lüdtke, A.; Wurr, S.; Rieger, T.; Muñoz-Fontela, C.; Günther, S. Successful Treatment of Advanced Ebola Virus Infection with T-705 (Favipiravir) in a Small Animal Model. Antivir. Res. 2014, 105, 17–21. [Google Scholar] [CrossRef]
- Parker, W.B. Metabolism and Antiviral Activity of Ribavirin. Virus Res. 2005, 107, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic Efficacy of the Small Molecule GS-5734 against Ebola Virus in Rhesus Monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Perwitasari, O.; Johnson, S.; Yan, X.; Howerth, E.; Shacham, S.; Landesman, Y.; Baloglu, E.; McCauley, D.; Tamir, S.; Tompkins, S.M.; et al. Verdinexor, a Novel Selective Inhibitor of Nuclear Export, Reduces Influenza a Virus Replication in Vitro and in Vivo. J. Virol. 2014, 88, 10228–10243. [Google Scholar] [CrossRef]
- Kinch, M.S.; Yunus, A.S.; Lear, C.; Mao, H.; Chen, H.; Fesseha, Z.; Luo, G.; Nelson, E.A.; Li, L.; Huang, Z.; et al. FGI-104: A Broad-Spectrum Small Molecule Inhibitor of Viral Infection. Am. J. Transl. Res. 2009, 1, 87–98. [Google Scholar] [CrossRef]
- Mentré, F.; Taburet, A.-M.; Guedj, J.; Anglaret, X.; Keïta, S.; de Lamballerie, X.; Malvy, D. Dose Regimen of Favipiravir for Ebola Virus Disease. Lancet Infect. Dis. 2015, 15, 150–151. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Guedj, J.; Anglaret, X.; Laouénan, C.; Madelain, V.; Taburet, A.-M.; Baize, S.; Sissoko, D.; Pastorino, B.; Rodallec, A.; et al. Favipiravir Pharmacokinetics in Ebola-Infected Patients of the JIKI Trial Reveals Concentrations Lower than Targeted. PLoS Negl. Trop. Dis. 2017, 11, e0005389. [Google Scholar] [CrossRef]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A New and Emerging Antiviral Option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Maidhof, A.; Taschner, H.; Zahn, R.K. Virazole (1-β-d-Ribofuranosyl-1,2,4-triazole-3-carboxamide; A Cytostatic Agent. Biochem. Pharmacol. 1977, 26, 1071–1075. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Kebriaei, R.; Dresser, L.D. Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19. Pharmacotherapy 2020, 40, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.C.; Freiberg, A.N.; Zhang, T.; Akyol-Ataman, Z.; Grock, A.; Hong, P.W.; Li, J.; Watson, N.F.; Fang, A.Q.; Aguilar, H.C.; et al. A Broad-Spectrum Antiviral Targeting Entry of Enveloped Viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Vigant, F.; Lee, J.; Hollmann, A.; Tanner, L.B.; Akyol Ataman, Z.; Yun, T.; Shui, G.; Aguilar, H.C.; Zhang, D.; Meriwether, D.; et al. A Mechanistic Paradigm for Broad-Spectrum Antivirals That Target Virus-Cell Fusion. PLoS Pathog. 2013, 9, e1003297. [Google Scholar] [CrossRef] [PubMed]
- Vigant, F.; Santos, N.C.; Lee, B. Broad-Spectrum Antivirals against Viral Fusion. Nat. Rev. Microbiol. 2015, 13, 426–437. [Google Scholar] [CrossRef]
- Palombi, N.; Brai, A.; Gerace, M.; Di Maria, S.; Orofino, F.; Corelli, F. Viral Envelope Membrane: A Special Entry Pathway and a Promising Drug Target. Curr. Med. Chem. 2021, 28, 6957–6976. [Google Scholar] [CrossRef] [PubMed]
- St. Vincent, M.R.; Colpitts, C.C.; Ustinov, A.V.; Muqadas, M.; Joyce, M.A.; Barsby, N.L.; Epand, R.F.; Epand, R.M.; Khramyshev, S.A.; Valueva, O.A.; et al. Rigid Amphipathic Fusion Inhibitors, Small Molecule Antiviral Compounds against Enveloped Viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 17339–17344. [Google Scholar] [CrossRef] [PubMed]
- Aralov, A.V.; Proskurin, G.V.; Orlov, A.A.; Kozlovskaya, L.I.; Chistov, A.A.; Kutyakov, S.V.; Karganova, G.G.; Palyulin, V.A.; Osolodkin, D.I.; Korshun, V.A. Perylenyltriazoles Inhibit Reproduction of Enveloped Viruses. Eur. J. Med. Chem. 2017, 138, 293–299. [Google Scholar] [CrossRef]
- Slesarchuk, N.A.; Khvatov, E.V.; Chistov, A.A.; Proskurin, G.V.; Nikitin, T.D.; Lazarevich, A.I.; Ulanovskaya, A.A.; Ulashchik, E.A.; Orlov, A.A.; Jegorov, A.V.; et al. Simplistic Perylene-Related Compounds as Inhibitors of Tick-Borne Encephalitis Virus Reproduction. Bioorg. Med. Chem. Lett. 2020, 30, 127100. [Google Scholar] [CrossRef]
- Vigant, F.; Hollmann, A.; Lee, J.; Santos, N.C.; Jung, M.E.; Lee, B. The Rigid Amphipathic Fusion Inhibitor DUY11 Acts through Photosensitization of Viruses. J. Virol. 2014, 88, 1849–1853. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Singh, U.K.; Singh, P.; Patel, R. Effect of N-Butyl-N-methyl-morpholinium Bromide Ionic Liquid on the Conformation Stability of Human Serum Albumin. ChemistrySelect 2017, 2, 1241–1249. [Google Scholar] [CrossRef]
- Pierra, C.; Amador, A.; Benzaria, S.; Cretton-Scott, E.; D’Amours, M.; Mao, J.; Mathieu, S.; Moussa, A.; Bridges, E.G.; Standring, D.N.; et al. Synthesis and Pharmacokinetics of Valopicitabine (Nm283), an Efficient Prodrug of the Potent Anti-Hcv Agent 2‘-C-Methylcytidine. J. Med. Chem. 2006, 49, 6614–6620. [Google Scholar] [CrossRef]
- Sigmundová, I.; Zahradník, P.; Magdolen, P.; Bujdáková, H. Synthesis and Study of New Antimicrobial Benzothiazoles Substituted on Heterocyclic Ring. Arkivoc 2008, 2008, 183–192. [Google Scholar] [CrossRef]
- Matsuda, A.; Takenuki, K.; Tanaka, M.; Sasaki, T.; Ueda, T. Nucleosides and Nucleotides. 97. Synthesis of New Broad Spectrum Antineoplastic Nucleosides, 2′-Deoxy-2′-methylidenecytidine (DMDC) and Its Derivatives. J. Med. Chem. 1991, 34, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Klochko, O.P.; Fedyunyayeva, I.A.; Khabuseva, S.U.; Semenova, O.M.; Terpetschnig, E.A.; Patsenker, L.D. Benzodipyrrolenine-Based Biscyanine Dyes: Synthesis, Molecular Structure and Spectroscopic Characterization. Dyes Pigm. 2010, 85, 7–15. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schäfer, A.; Dinnon, K.H.; Stevens, L.J.; et al. An Orally Bioavailable Broad-Spectrum Antiviral Inhibits SARS-CoV-2 in Human Airway Epithelial Cell Cultures and Multiple Coronaviruses in Mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef]
- Colpitts, C.C.; Ustinov, A.V.; Epand, R.F.; Epand, R.M.; Korshun, V.A.; Schang, L.M. 5-(Perylen-3-yl)ethynyl-arabino-uridine (AUY11), an Arabino-Based Rigid Amphipathic Fusion Inhibitor, Targets Virion Envelope Lipids to Inhibit Fusion of Influenza Virus, Hepatitis C Virus, and Other Enveloped Viruses. J. Virol. 2013, 87, 3640–3654. [Google Scholar] [CrossRef]
- Radchenko, E.V.; Dyabina, A.S.; Palyulin, V.A.; Zefirov, N.S. Prediction of Human Intestinal Absorption of Drug Compounds. Russ. Chem. Bull. 2016, 65, 576–580. [Google Scholar] [CrossRef]
- Dyabina, A.S.; Radchenko, E.V.; Palyulin, V.A.; Zefirov, N.S. Prediction of Blood-Brain Barrier Permeability of Organic Compounds. Dokl. Biochem. Biophys. 2016, 470, 371–374. [Google Scholar] [CrossRef]
- ADMET Prediction Service. Available online: http://Qsar.Chem.Msu.Ru/Admet/ (accessed on 26 August 2022).
- Chen, Q.; Li, P.; Li, S.; Xiao, W.; Yang, S.; Lu, H. Brain Complications with Influenza Infection in Children. Behav. Brain Sci. 2020, 10, 129–152. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-Term Neuroinflammation Induced by Influenza A Virus Infection and the Impact on Hippocampal Neuron Morphology and Function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Relevance of Half-Life in Drug Design: Miniperspective. J. Med. Chem. 2018, 61, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Zapata-Sudo, G.; da Costa Nunes, I.K.; Segundo Chaves de Araujo, J.; da Silva, J.; Manhães Trachez, M.; Fernandes da Silva, T.; da Costa, F.P.; Sudo, R.; Barreiro, E. Synthesis, Solubility, Plasma Stability, and Pharmacological Evaluation of Novel Sulfonylhydrazones Designed as Anti-Diabetic Agents. Drug Des. Devel. Ther. 2016, 10, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Hu, X.; Shi, X.; Wang, C.; Wang, L.; Wang, G. Pharmacokinetic, Metabolic Stability, Plasma Protein Binding and CYP450s Inhibition/Induction Assessment Studies of N-(2-Pyridylmethyl)-2-hydroxiymethyl-1-pyrrolidinyl-4-(3-chloro-4-methoxy-benzylamino)-5-pyrimidine-carboxamide as Potential Type 5 Phosphodiesterase Inhibitors. Anim. Cells Syst. 2019, 23, 155–163. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Bailey, K.W.; Bemis, P.A.; Wong, M.-H.; Eisenberg, E.J.; Huffman, J.H. Influence of Treatment Schedule and Viral Challenge Dose on the in Vivo Influenza Virus-Inhibitory Effects of the Orally Administered Neuraminidase Inhibitor GS 4104. Antivir. Chem. Chemother. 1999, 10, 187–193. [Google Scholar] [CrossRef]
- Govorkova, E.A.; Ilyushina, N.A.; McClaren, J.L.; Naipospos, T.S.P.; Douangngeun, B.; Webster, R.G. Susceptibility of Highly Pathogenic H5N1 Influenza Viruses to the Neuraminidase Inhibitor Oseltamivir Differs in Vitro and in a Mouse Model. Antimicrob. Agents Chemother. 2009, 53, 3088–3096. [Google Scholar] [CrossRef]
- Rakers, C.; Schwerdtfeger, S.-M.; Mortier, J.; Duwe, S.; Wolff, T.; Wolber, G.; Melzig, M.F. Inhibitory Potency of Flavonoid Derivatives on Influenza Virus Neuraminidase. Bioorg. Med. Chem. Lett. 2014, 24, 4312–4317. [Google Scholar] [CrossRef]
- Taleli, L.; de Kock, C.; Smith, P.J.; Pelly, S.C.; Blackie, M.A.L.; van Otterlo, W.A.L. In Vitro Antiplasmodial Activity of Triazole-Linked Chloroquinoline Derivatives Synthesized from 7-Chloro-N-(prop-2-yn-1-yl)quinolin-4-amine. Bioorg. Med. Chem. 2015, 23, 4163–4171. [Google Scholar] [CrossRef]
- Chen, X.; Peng, W.; Huang, S.; Yang, C.; Hu, M.; Yang, S.; Yang, S.; Xie, Y.; Chen, H.; Lei, N.; et al. Novel Mitochondria-Targeted and Fluorescent DNA Alkylation Agents with Highly Selective Activity against Cancer Cells. Dyes Pigm. 2019, 170, 107610. [Google Scholar] [CrossRef]
- Proskurin, G.V.; Orlov, A.A.; Brylev, V.A.; Kozlovskaya, L.I.; Chistov, A.A.; Karganova, G.G.; Palyulin, V.A.; Osolodkin, D.I.; Korshun, V.A.; Aralov, A.V. 3′-O-Substituted 5-(Perylen-3-ylethynyl)-2′-deoxyuridines as Tick-Borne Encephalitis Virus Reproduction Inhibitors. Eur. J. Med. Chem. 2018, 155, 77–83. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Kozlovskaya, L.I.; Volok, V.P.; Shtro, A.A.; Nikolaeva, Y.V.; Chistov, A.A.; Matyugina, E.S.; Belyaev, E.S.; Jegorov, A.V.; Snoeck, R.; Korshun, V.A.; et al. Phenoxazine Nucleoside Derivatives with a Multiple Activity against RNA and DNA Viruses. Eur. J. Med. Chem. 2021, 220, 113467. [Google Scholar] [CrossRef] [PubMed]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Konsoula, R.; Jung, M. In Vitro Plasma Stability, Permeability and Solubility of Mercaptoacetamide Histone Deacetylase Inhibitors. Int. J. Pharm. 2008, 361, 19–25. [Google Scholar] [CrossRef] [Green Version]



| Compound | Antiviral Activity, EC50 ± SD, µM | Cytotoxicity, CC50 ± SD, µM | |||
|---|---|---|---|---|---|
| CHIKV | SARS-CoV-2 | IAV | Vero | MDCK | |
| 5 d | 24 h | ||||
| 2a | 0.47 ± 0.16 | 11.3 ± 1.8 | 20.6 ± 8 | >100 | >100 |
| 2b | >100 | >100 | 62 ± 22 | >100 | >100 |
| 3a | 11.3 ± 9.0 | 1.88 ± 0.88 | 49 ± 19 | >100 | >100 |
| 3b | 10.7 ± 2.6 | 9.2 ± 4.3 | >100 | >100 | >100 |
| 5 | 1.17 ± 0.55 | >100 | ND | >100 | ND |
| 6 | >100 | >100 | 31 ± 10 | >100 | >100 |
| 7 | <0.016 | 0.075 ± 0.018 | 4.6 ± 1.0 | 98 ± 17 | 62 ± 24 |
| 8 | 0.55 ± 0.33 | 1.28 ± 0.40 | 3.8 ± 1.3 | >100 | >100 |
| 10 | 0.99 ± 0.15 | 1.45 ± 0.95 | 22.7 ± 10.0 | >100 | 69 ± 27 |
| Positive control | NHC a 9.7 ± 2.8 | NHC a 7.4 ± 3.6 | Tamiflu b 0.02 ± 0.01 | NHC a >100 | Tamiflu b >100 |
| Compound | ε, M−1·cm−1 | Solubility in Water, mM |
|---|---|---|
| 3a | 20,000 | 3.4 |
| 3b | 20,000 | 5.0 |
| 8 | 34,000 | 0.1 |
| 10 | 60,000 | 11.0 |
| aUY11 [37] | 41,000 | insoluble |
| Cmpd | Dose, mg/kg | Mortality Rate, % | p (Mortality Rates Comparison) | Average Life Expectancy (M ± SEM), days | p (Average Life Expectancy Comparison) | Protective Index (PI) |
|---|---|---|---|---|---|---|
| Placebo | – | 90 | – | 8.40 ± 0.9 | – | 0 |
| Tamiflu | 20 | 40 | 0.02084 * | 11.4 ± 1.1 | 0.0134 * | 56 |
| 10 | 4.5 | 70 | 0.7295 | 8.6 ± 1.2 | 0.4483 | 22 |
| 10 | 0.5 | 80 | 0.7362 | 8.6 ± 1.0 | 0.4524 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shtro, A.A.; Garshinina, A.V.; Alferova, V.A.; Kamzeeva, P.N.; Volok, V.P.; Kolpakova, E.S.; Nikitin, T.D.; Chistov, A.A.; Belyaev, E.S.; Korshun, V.A.; et al. Cationic Perylene Antivirals with Aqueous Solubility for Studies In Vivo. Pharmaceuticals 2022, 15, 1178. https://doi.org/10.3390/ph15101178
Shtro AA, Garshinina AV, Alferova VA, Kamzeeva PN, Volok VP, Kolpakova ES, Nikitin TD, Chistov AA, Belyaev ES, Korshun VA, et al. Cationic Perylene Antivirals with Aqueous Solubility for Studies In Vivo. Pharmaceuticals. 2022; 15(10):1178. https://doi.org/10.3390/ph15101178
Chicago/Turabian StyleShtro, Anna A., Anzhelika V. Garshinina, Vera A. Alferova, Polina N. Kamzeeva, Viktor P. Volok, Ekaterina S. Kolpakova, Timofei D. Nikitin, Alexey A. Chistov, Evgeny S. Belyaev, Vladimir A. Korshun, and et al. 2022. "Cationic Perylene Antivirals with Aqueous Solubility for Studies In Vivo" Pharmaceuticals 15, no. 10: 1178. https://doi.org/10.3390/ph15101178
APA StyleShtro, A. A., Garshinina, A. V., Alferova, V. A., Kamzeeva, P. N., Volok, V. P., Kolpakova, E. S., Nikitin, T. D., Chistov, A. A., Belyaev, E. S., Korshun, V. A., Kozlovskaya, L. I., & Aralov, A. V. (2022). Cationic Perylene Antivirals with Aqueous Solubility for Studies In Vivo. Pharmaceuticals, 15(10), 1178. https://doi.org/10.3390/ph15101178