Asian Pigeonwing Plants (Clitoria ternatea) Synergized Mesenchymal Stem Cells by Modulating the Inflammatory Response in Rats with Cisplatin-Induced Acute Kidney Injury
Abstract
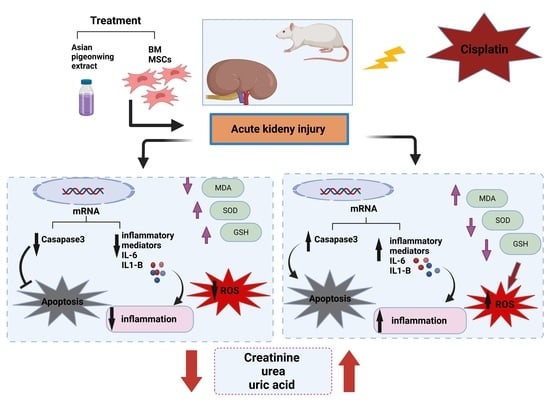
:1. Introduction
2. Results
2.1. Phytochemical Analysis
2.2. Kidney Functions
2.3. Oxidative Stress Markers
2.4. Gene Expression Profiling
2.5. Histopathological Sectioning
3. Discussion
3.1. Renal Functions
3.2. Renal Enzymes
4. Materials and Methods
4.1. Plant Sample Collection
4.2. Preparation of Aqueous Extract
4.3. Phytochemical Analysis and Assessment of Antioxidant Activity
4.4. Animals and Research Design
- The control group received saline.
- The cisplatin group was injected with a single dose of cisplatin of 7 mg/kg intraperitoneally (IP) to induce liver damage.
- The BM-MSC group (bone-marrow-derived MSCs group) was injected with a single dose of cisplatin of 7 mg/kg IP, and on the next day began receiving 2 × 106 BM-MSCs per day in phosphate buffer solution (PBS) by intravenous (IV) injection for 21 days.
- The Asian pigeonwing group was injected with a single dose of cisplatin of 7 mg/kg IP, and on the next day began receiving 400 mg/kg of Asian pigeonwing extract (by oral gavage for 21 days).
- The BM-MSC plus Asian pigeonwing group (combinational treatment group) was injected with a single dose of cisplatin of 7 mg/kg IP, and on the next day began receiving 2 × 106 BM-MSCs in PBS by IV injection plus 400 mg/kg/day of Asian pigeonwing by oral lavage for 21 days.
4.5. Stem Cells
4.6. Blood and Tissue Sampling
4.7. Biochemical Analyses
4.8. Histological Analysis
4.9. Gene Expression Analyses
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tseng, W.-C.; Lee, P.-Y.; Tsai, M.-T.; Chang, F.-P.; Chen, N.-J.; Chien, C.-T.; Hung, S.-C.; Tarng, D.-C. Hypoxic mesenchymal stem cells ameliorate acute kidney ischemia-reperfusion injury via enhancing renal tubular autophagy. Stem. Cell Res. Ther. 2021, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 52. [Google Scholar] [CrossRef]
- Mercado, M.G.; Smith, D.K.; Guard, E.L. Acute Kidney Injury: Diagnosis and Management. Am. Fam. Physician 2019, 100, 687–694. [Google Scholar]
- As, L.; Mt, J. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef]
- Mousa, D.; Alharbi, A.; Helal, I.; Al-homrany, M.; Alhujaili, F.; Alhweish, A.; Marie, M.A.; Al Sayyari, A. Prevalence and Associated Factors of Chronic Kidney Disease among Relatives of Hemodialysis Patients in Saudi Arabia. Kidney Int. Rep. 2021, 6, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, X.; Ding, X.; He, J.; Cai, G.; Zhu, H. Immunomodulatory Effects of Mesenchymal Stem Cells on Drug-Induced Acute Kidney Injury. Front. Immunol. 2021, 12, 683003. [Google Scholar] [CrossRef]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.-S.; Jiang, W.-P.; Chen, C.-C.; Lee, L.-Y.; Li, P.-Y.; Huang, W.-C.; Liao, J.-C.; Chen, H.-Y.; Huang, S.-S.; Huang, G.-J. Cordyceps cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF-κB/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice. Oxidative Med. Cell. Longev. 2020, 2020, 7912763. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem.-Biol. Interact. 2019, 308, 269–278. [Google Scholar] [CrossRef]
- Tan, R.-Z.; Liu, J.; Zhang, Y.-Y.; Wang, H.-L.; Li, J.-C.; Liu, Y.-H.; Zhong, X.; Zhang, Y.-W.; Yan, Y.; Lan, H.-Y.; et al. Curcumin relieved cisplatin-induced kidney inflammation through inhibiting Mincle-maintained M1 macrophage phenotype. Phytomedicine 2019, 52, 284–294. [Google Scholar] [CrossRef] [PubMed]
- ALshamrani, S.M.; Safhi, F.A.; Mobasher, M.A.; Saleem, R.M.; Alharthi, A.; Alshaya, D.S.; Awad, N.S. Antiproliferative Effect of Clitoria ternatea Ethanolic Extract against Colorectal, Breast, and Medullary Thyroid Cancer Cell Lines. Separations 2022, 9, 331. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. J. Food Sci. Technol. 2021, 58, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Kumar, N.S.; Heinrich, M. The Ayurvedic medicine Clitoria ternatea—From traditional use to scientific assessment. J. Ethnopharmacol. 2008, 120, 291–301. [Google Scholar] [CrossRef]
- Peired, A.J.; Sisti, A.; Romagnani, P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016, 2016, 4798639. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Kong, Y.; Xie, C.; Zhou, L. Stem/progenitor cell in kidney: Characteristics, homing, coordination, and maintenance. Stem Cell Res. Ther. 2021, 12, 197. [Google Scholar] [CrossRef]
- Tsuji, K.; Kitamura, S.; Wada, J. Secretomes from Mesenchymal Stem Cells against Acute Kidney Injury: Possible Heterogeneity. Stem Cells Int. 2018, 2018, 8693137. [Google Scholar] [CrossRef] [Green Version]
- Mobasher, M.A.; Valverde, Á.M. Signalling pathways involved in paracetamol-induced hepatotoxicity: New insights on the role of protein tyrosine phosphatase 1B. Arch. Physiol. Biochem. 2014, 120, 51–63. [Google Scholar] [CrossRef]
- Lee, P.-W.; Wu, B.-S.; Yang, C.-Y.; Lee, O.K.-S. Molecular Mechanisms of Mesenchymal Stem Cell-Based Therapy in Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 11406. [Google Scholar] [CrossRef]
- Almaeen, A. H.; Alduraywish, A. A.; Mobasher, M. A.; Almadhi, O.; Nafeh, H. M.; El-Metwally, T. H. Oxidative stress, immunological and cellular hypoxia biomarkers in hepatitis C treatment-naïve and cirrhotic patients. Arch. Med. Sci. AMS 2020, 17, 368–375. [Google Scholar] [CrossRef]
- Saud, B.; Malla, R.; Shrestha, K. A Review on the Effect of Plant Extract on Mesenchymal Stem Cell Proliferation and Differentiation. Stem Cells Int. 2019, 2019, 7513404. [Google Scholar] [CrossRef] [PubMed]
- Kindgen-Milles, D.; Slowinski, T.; Dimski, T. New kidney function tests: Renal functional reserve and furosemide stress test. Med. Klin. Intensivmed. Notfmed. 2020, 115, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.I.; Bajo, M.; Marti, F.; Musso, C.G. How to evaluate renal function in stable cirrhotic patients. Postgrad. Med. 2017, 129, 866–871. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, S. A; El-Said, K. S.; Mobasher, M.; Elbakry, M. Enhancing antitumor efficacy of cisplatin low dose by EDTA in Ehrlich ascetic carcinoma bearing mice. Braz. Arch. Biol. Technol. 2019, 62, e19180716. [Google Scholar] [CrossRef]
- Li, X.; Liao, J.; Su, X.; Li, W.; Bi, Z.; Wang, J.; Su, Q.; Huang, H.; Wei, Y.; Gao, Y.; et al. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics 2020, 10, 9561–9578. [Google Scholar] [CrossRef]
- Maneesai, P.; Iampanichakul, M.; Chaihongsa, N.; Poasakate, A.; Potue, P.; Rattanakanokchai, S.; Bunbupha, S.; Chiangsaen, P.; Pakdeechote, P. Butterfly Pea Flower (Clitoria ternatea Linn.) Extract Ameliorates Cardiovascular Dysfunction and Oxidative Stress in Nitric Oxide-Deficient Hypertensive Rats. Antioxidants 2021, 10, 523. [Google Scholar] [CrossRef]
- Chung, M.-J.; Son, J.-Y.; Park, S.; Park, S.-S.; Hur, K.; Lee, S.-H.; Lee, E.-J.; Park, J.-K.; Hong, I.-H.; Kim, T.-H.; et al. Mesenchymal Stem Cell and MicroRNA Therapy of Musculoskeletal Diseases. Int. J. Stem Cells 2020, 14, 150–167. [Google Scholar] [CrossRef]
- Yang, C.; Luo, M.; Chen, Y.; You, M.; Chen, Q. MicroRNAs as Important Regulators Mediate the Multiple Differentiation of Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2021, 9, 619842. [Google Scholar] [CrossRef]
- Cao, J.-Y.; Wang, B.; Tang, T.-T.; Wen, Y.; Li, Z.-L.; Feng, S.-T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.-L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, C.; Xian, S.; Zhuang, F.; Ding, F.; Zhang, W.; Liu, Y. Placental Mesenchymal Stromal Cells (PMSCs) and PMSC-Derived Extracellular Vesicles (PMSC-EVs) Attenuated Renal Fibrosis in Rats with Unilateral Ureteral Obstruction (UUO) by Regulating CD4+ T Cell Polarization. Stem Cells Int. 2020, 2020, 2685820. [Google Scholar] [CrossRef]
- Guo, J.; Wang, R.; Liu, D. Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate Sepsis-Induced Acute Kidney Injury by Promoting Mitophagy of Renal Tubular Epithelial Cells via the SIRT1/Parkin Axis. Front. Endocrinol. 2021, 12, 639165. [Google Scholar] [CrossRef] [PubMed]
- Matsui, F.; Babitz, S.K.; Rhee, A.; Hile, K.L.; Zhang, H.; Meldrum, K.K. Mesenchymal stem cells protect against obstruction-induced renal fibrosis by decreasing STAT3 activation and STAT3-dependent MMP-9 production. Am. J. Physiol. Renal. Physiol. 2017, 312, F25–F32. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. Potential and Therapeutic Efficacy of Cell-based Therapy Using Mesenchymal Stem Cells for Acute/chronic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chayaratanasin, P.; Adisakwattana, S.; Thilavech, T. Protective role of Clitoria ternatea L. flower extract on methylglyoxal-induced protein glycation and oxidative damage to DNA. BMC Complement. Med. Ther. 2021, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Gamage, V.G.C.; Yy, L.; Ws, C. Anthocyanins From Clitoria ternatea Flower: Biosynthesis, Extraction, Stability, Antioxidant Activity, and Applications. Front. Plant Sci. 2021, 12, 792303. [Google Scholar] [CrossRef] [PubMed]
- Saengnak, B.; Kanla, P.; Samrid, R.; Berkban, T.; Mothong, W.; Pakdeechote, P.; Prachaney, P. Clitoria ternatea L. extract prevents kidney damage by suppressing the Ang II/Nox4/oxidative stress cascade in l-NAME-induced hypertension model of rats. Ann. Anat. 2021, 238, 151783. [Google Scholar] [CrossRef]
- Al Shukor, N.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-Converting Enzyme Inhibitory Effects by Plant Phenolic Compounds: A Study of Structure Activity Relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef]
- Bunbupha, S.; Apaijit, K.; Potue, P.; Maneesai, P.; Pakdeechote, P. Hesperidin inhibits L-NAME-induced vascular and renal alterations in rats by suppressing the renin–angiotensin system, transforming growth factor-β1, and oxidative stress. Clin. Exp. Pharmacol. Physiol. 2021, 48, 412–421. [Google Scholar] [CrossRef]
- AngII-Induced Glomerular Mesangial Cell Proliferation Inhibited by Losartan via Changes in Intracellular Calcium Ion Concentration—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4000622/ (accessed on 27 October 2022).
- Sachse, A.; Wolf, G. Angiotensin II-induced reactive oxygen species and the kidney. J. Am. Soc. Nephrol. 2007, 18, 2439–2446. [Google Scholar] [CrossRef] [Green Version]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [Green Version]
- Festa, M.; Sansone, C.; Brunet, C.; Crocetta, F.; Di Paola, L.; Lombardo, M.; Bruno, A.; Noonan, D.M.; Albini, A. Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARS-CoV-2 Infection. Int. J. Mol. Sci. 2020, 21, 8364. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky, T.A. Evaluation of effectiveness aqueous extract for some leaves of wild edible plants in Egypt as anti-fungal and anti-toxigenic. Heliyon 2021, 7, e06209. [Google Scholar] [CrossRef] [PubMed]
- Koldaş, S.; Demirtas, I.; Ozen, T.; Demirci, M.A.; Behçet, L. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J. Sci. Food Agric. 2015, 95, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sahu, R.; Saxena, J. Screening of Total Phenolic and Flavonoid Content in Conventional and Non-Conventional Species of Curcuma. J. Pharmacogn. Phytochem. 2013, 2, 176–179. [Google Scholar]
- Puranik, A.S.; Leaf, I.A.; Jensen, M.A.; Hedayat, A.F.; Saad, A.; Kim, K.-W.; Saadalla, A.M.; Woollard, J.R.; Kashyap, S.; Textor, S.C.; et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci. Rep. 2018, 8, 13948. [Google Scholar] [CrossRef] [PubMed]






| Total phenol (mg/g extract) | 31.3 |
| Flavonoid (mg/g extract, as quercetin) | 5.2 |
| FRAP assay reduction power (mg/g extract, as ascorbic acid) | 25.8 |
| Genes | Forward (5′–3′) | Reverse (5′–3′) | Size |
|---|---|---|---|
| IL-1β | CAGCAGCATCTCGACAAGAG | AAAGAAGGTGCTTGGGTCCT | 123 bp |
| IL-6 | AGTTGCCTTCTTGGGACTGA | CCTCCGACTTGTGAAGTGGT | 126 bp |
| Caspase-3 | GAGACAGACAGTGGAACTGACGA TG | GGCGCAAAGTGACTGGATGA | 147 bp |
| β-Actin | GTGACATCCACACCCAGAGG | ACAGGATGTCAAAACTGCCC- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safhi, F.A.; ALshamrani, S.M.; Jalal, A.S.; Awad, N.S.; Sabit, H.; Abdelgawad, F.E.; Khalil, S.S.; Khodeer, D.M.; Mobasher, M.A. Asian Pigeonwing Plants (Clitoria ternatea) Synergized Mesenchymal Stem Cells by Modulating the Inflammatory Response in Rats with Cisplatin-Induced Acute Kidney Injury. Pharmaceuticals 2022, 15, 1396. https://doi.org/10.3390/ph15111396
Safhi FA, ALshamrani SM, Jalal AS, Awad NS, Sabit H, Abdelgawad FE, Khalil SS, Khodeer DM, Mobasher MA. Asian Pigeonwing Plants (Clitoria ternatea) Synergized Mesenchymal Stem Cells by Modulating the Inflammatory Response in Rats with Cisplatin-Induced Acute Kidney Injury. Pharmaceuticals. 2022; 15(11):1396. https://doi.org/10.3390/ph15111396
Chicago/Turabian StyleSafhi, Fatmah A., Salha M. ALshamrani, Areej S. Jalal, Nabil S. Awad, Hussein Sabit, Fathy Elsayed Abdelgawad, Sama S. Khalil, Dina M. Khodeer, and Maysa A. Mobasher. 2022. "Asian Pigeonwing Plants (Clitoria ternatea) Synergized Mesenchymal Stem Cells by Modulating the Inflammatory Response in Rats with Cisplatin-Induced Acute Kidney Injury" Pharmaceuticals 15, no. 11: 1396. https://doi.org/10.3390/ph15111396
APA StyleSafhi, F. A., ALshamrani, S. M., Jalal, A. S., Awad, N. S., Sabit, H., Abdelgawad, F. E., Khalil, S. S., Khodeer, D. M., & Mobasher, M. A. (2022). Asian Pigeonwing Plants (Clitoria ternatea) Synergized Mesenchymal Stem Cells by Modulating the Inflammatory Response in Rats with Cisplatin-Induced Acute Kidney Injury. Pharmaceuticals, 15(11), 1396. https://doi.org/10.3390/ph15111396