PfMDR1 Transport Rates Assessed in Intact Isolated Plasmodium falciparum Digestive Vacuoles Reflect Functional Drug Resistance Relationship with pfmdr1 Mutations
Abstract
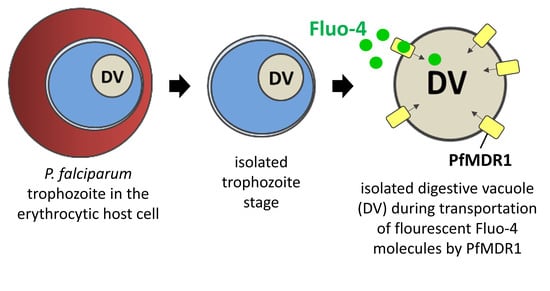
:1. Introduction

2. Results and Discussion
2.1. Surface Area of Isolated Digestive Vacuoles
2.2. Estimated PfMDR1 Transporter DV Surface Expression
2.3. Fluo-4 Uptake by PfMDR1 in Isolated DVs
3. Materials and Methods
3.1. Cultivation and Synchronization of Malaria Parasites
3.2. Isolation of Trophozoites and Digestive Vacuoles from Infected Red Blood Cells (iRBC)
3.3. Immunofluorescence Labelling of Isolated DVs and Diameter Measurements
3.4. Recombinant PfMDR1-c-Terminal-His Protein Production
3.5. ELISA for the Detection of Recombinant and Endogenous PfMDR1
3.6. Fluo-4 Uptake Assays with Isolated DVs
4. Conclusions
- Viable DVs from parasite trophozoite stages of RBCs infected with multi-drug resistant and sensitive strains can be obtained with an optimized bio-separation strategy described here.
- DVs isolated in this manner contain functional PfMDR1 transporters that are responsive to both ATP and drugs and can be used for ‘reverse Fluo-4 Ca2+ imaging’ to monitor PfMDR1 transport rates in the isolated compartment, free of cytoplasmic influences of the parasite.
- Amino acid exchanges due to mutations in the pfmdr1 gene might lead to higher Fluo-4 transport rates of the PfMDR1 transport protein
- Isolated DVs can be subjected to a high-content assay that, alongside with morphometric analyses of DV surface area and quantitative ELISA for PfMDR1 binding studies, provide the unique opportunity to relate global transport rates from whole DVs to molecular PfMDR1 rates.
- The high content transport rate assay described here will be of high value for upcoming in vitro drug screening assays, as DVs can be stably stored following the bio-separation process and allow a constant sample quality.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- WHO. World Malaria Report; WHO: Geneva, Switzerland, 2020.
- Capci, A.; Lorion, M.M.; Wang, H.; Simon, N.; Leidenberger, M.; Borges Silva, M.C.; Moreira, D.R.; Zhu, Y.; Meng, Y.; Chen, J.Y.; et al. Artemisinin-(Iso)quinoline Hybrids by C-H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria. Angew. Chem. Int. Ed. Engl. 2019, 58, 13066–13079. [Google Scholar] [CrossRef] [Green Version]
- Quadros, H.C.; Capci, A.; Herrmann, L.; D’Alessandro, S.; Fontinha, D.; Azevedo, R.; Villarreal, W.; Basilico, N.; Prudêncio, M.; Tsogoeva, S.B.; et al. Studies of Potency and Efficacy of an Optimized Artemisinin-Quinoline Hybrid against Multiple Stages of the Plasmodium Life Cycle. Pharmaceuticals 2021, 14, 1129. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [Green Version]
- Makler, M.T.; Hinrichs, D.J. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 1993, 48, 205–210. [Google Scholar] [CrossRef]
- Carolino, K.; Winzeler, E.A. The antimalarial resistome—Finding new drug targets and their modes of action. Curr. Opin. Microbiol. 2020, 57, 49–55. [Google Scholar] [CrossRef]
- de Villiers, K.A.; Egan, T.J. Heme Detoxification in the Malaria Parasite: A Target for Antimalarial Drug Development. Acc. Chem. Res. 2021, 54, 2649–2659. [Google Scholar] [CrossRef]
- Cowman, A.F.; Karcz, S.; Galatis, D.; Culvenor, J.G. A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J. Cell Biol. 1991, 113, 1033–1042. [Google Scholar] [CrossRef]
- Peel, S.A. The ABC transporter genes of Plasmodium falciparum and drug resistance. Drug Resist. Updat. 2001, 4, 66–74. [Google Scholar] [CrossRef]
- Rohrbach, P.; Sanchez, C.P.; Hayton, K.; Friedrich, O.; Patel, J.; Sidhu, A.B.; Ferdig, M.T.; Fidock, D.A.; Lanzer, M. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. EMBO J. 2006, 25, 3000–3011. [Google Scholar] [CrossRef] [Green Version]
- Lehne, G. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr. Drug Targets 2000, 1, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, O.; Reiling, S.J.; Wunderlich, J.; Rohrbach, P. Assessment of Plasmodium falciparum PfMDR1 transport rates using Fluo-4. J. Cell. Mol. Med. 2014, 18, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Matz, J.M.; Beck, J.R.; Blackman, M.J. The parasitophorous vacuole of the blood-stage malaria parasite. Nat. Rev. Microbiol. 2020, 18, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.C.; Ubben, D.; Wells, T.N. A framework for assessing the risk of resistance for anti-malarials in development. Malar J. 2012, 11, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chugh, M.; Scheurer, C.; Sax, S.; Bilsland, E.; van Schalkwyk, D.A.; Wicht, K.J.; Hofmann, N.; Sharma, A.; Bashyam, S.; Singh, S.; et al. Identification and deconvolution of cross-resistance signals from antimalarial compounds using multidrug-resistant Plasmodium falciparum strains. Antimicrob. Agents Chemother. 2015, 59, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Nzila, A.; Mwai, L. In vitro selection of Plasmodium falciparum drug-resistant parasite lines. J. Antimicrob. Chemother. 2010, 65, 390–398. [Google Scholar] [CrossRef]
- Veiga, M.I.; Dhingra, S.K.; Henrich, P.P.; Straimer, J.; Gnadig, N.; Uhlemann, A.C.; Martin, R.E.; Lehane, A.M.; Fidock, D.A. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat. Commun. 2016, 7, 11553. [Google Scholar] [CrossRef] [Green Version]
- Rohrbach, P.; Friedrich, O.; Hentschel, J.; Plattner, H.; Fink, R.H.; Lanzer, M. Quantitative calcium measurements in subcellular compartments of Plasmodium falciparum-infected erythrocytes. J. Biol. Chem. 2005, 280, 27960–27969. [Google Scholar] [CrossRef] [Green Version]
- Reiling, S.J.; Krohne, G.; Friedrich, O.; Geary, T.G.; Rohrbach, P. Chloroquine exposure triggers distinct cellular responses in sensitive versus resistant Plasmodium falciparum parasites. Sci. Rep. 2018, 8, 11137. [Google Scholar] [CrossRef] [Green Version]
- Hamid, E.; Church, E.; Alford, S. Quantitation and Simulation of Single Action Potential-Evoked Ca(2+) Signals in CA1 Pyramidal Neuron Presynaptic Terminals. eNeuro 2019, 6, 0343-19. [Google Scholar] [CrossRef]
- Reiling, S.J.; Rohrbach, P. Monitoring PfMDR1 transport in Plasmodium falciparum. Malar J. 2015, 14, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Kehr, S.; Sturm, N.; Rahlfs, S.; Przyborski, J.M.; Becker, K. Compartmentation of redox metabolism in malaria parasites. PLoS Pathog. 2010, 6, e1001242. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.L.; González, D.A.; Takara, D.; Ostuni, M.A.; Sánchez, G.A. Calcium additional to that bound to the transport sites is required for full activation of the sarcoplasmic reticulum Ca-ATPase from skeletal muscle. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 1998, 1405, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Buxbaum, E. Co-operating ATP sites in the multiple drug resistance transporter Mdr1. Eur. J. Biochem. 1999, 265, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koley, D.; Bard, A.J. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783–16787. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, N.; Voigtländer, C.; Kappes, B.; Rohrbach, P.; Friedrich, O. PfMDR1 Transport Rates Assessed in Intact Isolated Plasmodium falciparum Digestive Vacuoles Reflect Functional Drug Resistance Relationship with pfmdr1 Mutations. Pharmaceuticals 2022, 15, 202. https://doi.org/10.3390/ph15020202
Simon N, Voigtländer C, Kappes B, Rohrbach P, Friedrich O. PfMDR1 Transport Rates Assessed in Intact Isolated Plasmodium falciparum Digestive Vacuoles Reflect Functional Drug Resistance Relationship with pfmdr1 Mutations. Pharmaceuticals. 2022; 15(2):202. https://doi.org/10.3390/ph15020202
Chicago/Turabian StyleSimon, Nina, Cornelia Voigtländer, Barbara Kappes, Petra Rohrbach, and Oliver Friedrich. 2022. "PfMDR1 Transport Rates Assessed in Intact Isolated Plasmodium falciparum Digestive Vacuoles Reflect Functional Drug Resistance Relationship with pfmdr1 Mutations" Pharmaceuticals 15, no. 2: 202. https://doi.org/10.3390/ph15020202
APA StyleSimon, N., Voigtländer, C., Kappes, B., Rohrbach, P., & Friedrich, O. (2022). PfMDR1 Transport Rates Assessed in Intact Isolated Plasmodium falciparum Digestive Vacuoles Reflect Functional Drug Resistance Relationship with pfmdr1 Mutations. Pharmaceuticals, 15(2), 202. https://doi.org/10.3390/ph15020202