The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study
Abstract
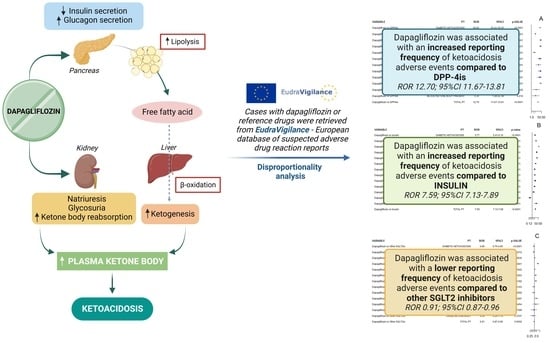
:1. Introduction
2. Results
2.1. ROR in General Diabetes
2.2. ROR in T1DM
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Design
4.2. Data Source
4.3. ICSRs Selection with Line Listing
4.4. Descriptive Analyses
4.5. Descriptive Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plosker, G.L. Dapagliflozin: A Review of Its Use in Patients with Type 2 Diabetes. Drugs 2014, 74, 2191–2209. [Google Scholar] [CrossRef] [PubMed]
- Vivian, E.M. Dapagliflozin: A new sodium–glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. Am. J. Health Pharm. 2015, 72, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Sundram, K.; Dhanaraj, S.A. Dapagliflozin: Glucuretic action and beyond. Pharmacol. Res. 2014, 82, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Scavone, C.; Scisciola, L.; Chiodini, P.; Capuano, A.; Paolisso, G. SGLT-2 inhibitors reduce the risk of cerebrovascular/cardiovascular outcomes and mortality: A systematic review and meta-analysis of retrospective cohort studies. Pharmacol. Res. 2021, 172, 105836. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Type 2 Diabetes in Adults: Management. NICE Guideline. Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 17 February 2022).
- Paik, J.; Blair, H.A. Dapagliflozin: A Review in Type 1 Diabetes. Drugs 2019, 79, 1877–1884. [Google Scholar] [CrossRef] [Green Version]
- HPRA. Forxiga (Dapagliflozin) 5 mg Should No Longer Be Used for the Treatment of Type 1 Diabetes Mellitus. Available online: https://www.hpra.ie/docs/default-source/default-document-library/important-safety-information-forxiga-(dapagliflozin)-5mg.pdf?sfvrsn=0 (accessed on 3 November 2021).
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Griffen, S.C.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M.; Proietto, J.; et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 864–876. [Google Scholar] [CrossRef]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M.; Proietto, J.; Stranks, S.; et al. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care 2018, 41, 2552–2559. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, C.; Dandona, P.; Gillard, P.; Senior, P.; Hasslacher, C.; Araki, E.; Lind, M.; Bain, S.C.; Jabbour, S.; Arya, N.; et al. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes (the DEPICT-2 Study): 24-Week Results From a Randomized Controlled Trial. Diabetes Care 2018, 41, 1938–1946. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, C.; Rudofsky, G.; Phillip, M.; Araki, E.; Lind, M.; Arya, N.; Thorén, F.; Scheerer, M.F.; Iqbal, N.; Dandona, P. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT -2 study): 52-week results from a randomized controlled trial. Diabetes Obes. Metab. 2020, 22, 1516–1526. [Google Scholar] [CrossRef]
- PRAC. SGLT2 Inhibitors: PRAC Makes Recommendations to Minimise Risk of Diabetic Ketoacidosis|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/sglt2-inhibitors-prac-makes-recommendations-minimise-risk-diabetic-ketoacidosis (accessed on 3 November 2021).
- FDA. Rejects Dapagliflozin as Treatment Add-On For Type 1 Diabetes–Diabetes. Available online: https://www.diabetes.co.uk/news/2019/jul/fda-rejects-dapagliflozin-as-treatment-add-on-for-type-1-diabetes-98751641.html (accessed on 25 November 2021).
- Goldenberg, R.M.; Gilbert, J.D.; Hramiak, I.M.; Woo, V.C.; Zinman, B. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: The STOP DKA Protocol. Diabete. Obes. Metab. 2019, 21, 2192–2202. [Google Scholar] [CrossRef]
- Goldenberg, R.M.; Berard, L.D.; Cheng, A.Y.; Gilbert, J.D.; Verma, S.; Woo, V.C.; Yale, J.-F. SGLT2 Inhibitor–associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin. Ther. 2016, 38, 2654–2664.e1. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.R.; Schumacher, C.; Harpe, S.E. SGLT2 Inhibitors: A Systematic Review of Diabetic Ketoacidosis and Related Risk Factors in the Primary Literature. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Danne, T.; Garg, S.; Peters, A.L.; Buse, J.B.; Mathieu, C.; Pettus, J.H.; Alexander, C.M.; Battelino, T.; Ampudia-Blasco, F.J.; Bode, B.W.; et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients with Type 1 Diabetes Treated With Sodium–Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care 2019, 42, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Novikov, A.; Vallon, V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes Metab. Res. Rev. 2017, 33, e2886. [Google Scholar] [CrossRef]
- Douros, A.; Lix, L.M.; Fralick, M.; Dell’Aniello, S.; Shah, B.R.; Ronksley, P.E.; Tremblay, É.; Hu, N.; Alessi-Severini, S.; Fisher, A.; et al. Sodium–Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis: A Multicenter Cohort Study. Ann. Intern. Med. 2020, 173, 417–425. [Google Scholar] [CrossRef]
- McGurnaghan, S.J.; on behalf of the Scottish Diabetes Research Network Epidemiology Group; Brierley, L.; Caparrotta, T.M.; McKeigue, P.M.; Blackbourn, L.A.K.; Wild, S.H.; Leese, G.P.; McCrimmon, R.J.; McKnight, J.A.; et al. The effect of dapagliflozin on glycaemic control and other cardiovascular disease risk factors in type 2 diabetes mellitus: A real-world observational study. Diabetologia 2019, 62, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Ueda, P.; Svanström, H.; Melbye, M.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Pasternak, B. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: Nationwide register based cohort study. BMJ 2018, 363, k4365. [Google Scholar] [CrossRef] [Green Version]
- Blau, J.E.; Tella, S.H.; Taylor, S.I.; Rother, K.I. Ketoacidosis associated with SGLT2 inhibitor treatment: Analysis of FAERS data. Diabetes/Metab. Res. Rev. 2017, 33, e2924. [Google Scholar] [CrossRef]
- Scavone, C.; Di Mauro, C.; Ruggiero, R.; Bernardi, F.F.; Trama, U.; Aiezza, M.L.; Rafaniello, C.; Capuano, A. Severe Cutaneous Adverse Drug Reactions Associated with Allopurinol: An Analysis of Spontaneous Reporting System in Southern Italy. Drugs Real World Outcomes 2020, 7, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Mascolo, A.; Ruggiero, R.; Sessa, M.; Scavone, C.; Sportiello, L.; Rafaniello, C.; Rossi, F.; Capuano, A. Preventable Cases of Oral Anticoagulant-Induced Bleeding: Data From the Spontaneous Reporting System. Front. Pharmacol. 2019, 10, 425. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, G.; Zinzi, A.; Vitiello, F.; Restaino, M.; Sportiello, L.; Rafaniello, C.; Sullo, M.; Capuano, A. Adverse drug reactions and gender differences: What changes in drug safety? Ital. J. Gend. Specif. Med. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Ema Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf (accessed on 4 November 2021).
- di Mauro, G.; Zinzi, A.; Scavone, C.; Mascolo, A.; Gaio, M.; Sportiello, L.; Ferrajolo, C.; Rafaniello, C.; Rossi, F.; Capuano, A. PCSK9 Inhibitors and Neurocognitive Adverse Drug Reactions: Analysis of Individual Case Safety Reports from the Eudravigilance Database. Drug Saf. 2021, 44, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.; Gaio, M.; Zinzi, A.; Scavone, C.; Gargano, F.; Coscioni, E.; Rossi, F.; Capuano, A. Cardiac Events Potentially Associated to Remdesivir: An Analysis from the European Spontaneous Adverse Event Reporting System. Pharmaceuticals 2021, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Scavone, C.; Ferrajolo, C.; Rafaniello, C.; Danesi, R.; Del Re, M.; Russo, A.; Coscioni, E.; Rossi, F.; Alfano, R.; et al. Immune Checkpoint Inhibitors and Cardiotoxicity: An Analysis of Spontaneous Reports in Eudravigilance. Drug Saf. 2021, 44, 957–971. [Google Scholar] [CrossRef]
- Sessa, M.; Rafaniello, C.; Scavone, C.; Mascolo, A.; Di Mauro, G.; Fucile, A.; Rossi, F.; Sportiello, L.; Capuano, A. Preventable statin adverse reactions and therapy discontinuation. What can we learn from the spontaneous reporting system? Expert Opin. Drug Saf. 2018, 17, 457–465. [Google Scholar] [CrossRef]
- Scavone, C.; Di Mauro, C.; Brusco, S.; Bertini, M.; Di Mauro, G.; Rafaniello, C.; Sportiello, L.; Rossi, F.; Capuano, A. Surveillance of adverse events following immunization related to human papillomavirus vaccines: 12 years of vaccinovigilance in Southern Italy. Expert Opin. Drug Saf. 2019, 18, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.L.; McGuire, D.K.; Danne, T.; Kushner, J.A.; Rodbard, H.W.; Dhatariya, K.; Sawhney, S.; Banks, P.; Jiang, W.; Davies, M.J.; et al. Diabetic Ketoacidosis and Related Events with Sotagliflozin Added to Insulin in Adults with Type 1 Diabetes: A Pooled Analysis of the inTandem 1 and 2 Studies. Diabetes Care 2020, 43, 2713–2720. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, X.; Guo, X.; Liu, D.; Xu, J.; Hu, F.; Zhai, Y.; Gao, Y.; Xu, X.; Dong, Z.; et al. Safety of SGLT2 Inhibitors: A Pharmacovigilance Study from 2013 to 2021 Based on FAERS. Front. Pharmacol. 2021, 12, 3618. [Google Scholar] [CrossRef]
- Katsuhara, Y.; Ogawa, T. Acute Renal Failure, Ketoacidosis, and Urogenital Tract Infections with SGLT2 Inhibitors: Signal Detection Using a Japanese Spontaneous Reporting Database. Clin. Drug Investig. 2020, 40, 645–652. [Google Scholar] [CrossRef]
- Colacci, M.; Fralick, J.; Odutayo, A.; Fralick, M. Sodium-Glucose Cotransporter-2 Inhibitors and Risk of Diabetic Ketoacidosis Among Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Can. J. Diabetes 2021, 46, 10–15.e2. [Google Scholar] [CrossRef]
- Taylor, S.I.; Blau, J.E.; Rother, K.I. SGLT2 Inhibitors May Predispose to Ketoacidosis. J. Clin. Endocrinol. Metab. 2015, 100, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, I.; Hamid, M.; Khan, M.A.A.; Kainat, A.; Tariq, S. Dapagliflozin-induced Late-onset Euglycemic Diabetic Ketoacidosis. Cureus 2019, 11, e6089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujara, S.; Ioachimescu, A. Prolonged Ketosis in a Patient with Euglycemic Diabetic Ketoacidosis Secondary to Dapagliflozin. J. Investig. Med. High Impact Case Rep. 2017, 5, 5. [Google Scholar] [CrossRef]
- Phillip, M.; Mathieu, C.; Lind, M.; Araki, E.; di Bartolo, P.; Bergenstal, R.; Heller, S.; Hansen, L.; Scheerer, M.F.; Thoren, F.; et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: Pooled 52-week outcomes from the DEPICT -1 and -2 studies. Diabete. Obes. Metab. 2021, 23, 549–560. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, Z.; Wei, Y. Efficacy and safety of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: A meta-analysis of randomized controlled trials. Exp. Ther. Med. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Eledrisi, M.S.; Elzouki, A.N. Management of Diabetic Ketoacidosis in Adults: A Narrative Review. Saudi J. Med. Med. Sci. 2020, 8, 165–173. [Google Scholar] [CrossRef]
- Wang, K.M.; Isom, R.T. SGLT2 Inhibitor–Induced Euglycemic Diabetic Ketoacidosis: A Case Report. Kidney Med. 2020, 2, 218–221. [Google Scholar] [CrossRef]
- Bader, N.; Mirza, L. Euglycemic Diabetic Ketoacidosis in a 27 year-old female patient with type-1-Diabetes treated with sodium-glucose cotransporter-2 (SGLT2) inhibitor Canagliflozin. Pak. J. Med. Sci. 2016, 32, 786–788. [Google Scholar] [CrossRef]
- Steinmetz-Wood, S.; Gilbert, M.; Menson, K. A Case of Diabetic Ketoacidosis in a Patient on an SGLT2 Inhibitor and a Ketogenic Diet: A Critical Trio Not to Be Missed. Case Rep. Endocrinol. 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- Milder, T.Y.; Stocker, S.L.; Baysari, M.; Day, R.O.; Greenfield, J.R. Prescribing of SGLT2 inhibitors in primary care: A qualitative study of General Practitioners and Endocrinologists. Diabetes Res. Clin. Pract. 2021, 180, 109036. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Vaduganathan, M. Practical Guide to Prescribing Sodium-Glucose Cotransporter 2 Inhibitors for Cardiologists. JACC Hear. Fail. 2019, 7, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Peterson, E.; Pagidipati, N. Barriers to prescribing glucose-lowering therapies with cardiometabolic benefits. Am. Hear. J. 2020, 224, 47–53. [Google Scholar] [CrossRef] [PubMed]
- EMA. SGLT2 Inhibitors Article 20–PRAC Assessment Report. 2016. Available online: https://www.ema.europa.eu/en/documents/referral/sglt2-inhibitors-article-20-procedure-assessment-report_en.pdf (accessed on 21 September 2021).
- Ruggiero, R.; Fraenza, F.; Scavone, C.; Di Mauro, G.; Piscitelli, R.; Mascolo, A.; Ferrajolo, C.; Rafaniello, C.; Sportiello, L.; Rossi, F.; et al. Immune Checkpoint Inhibitors and Immune-Related Adverse Drug Reactions: Data from Italian Pharmacovigilance Database. Front. Pharmacol. 2020, 11, 830. [Google Scholar] [CrossRef]
- Fralick, M.; Schneeweiss, S.; Patorno, E. Risk of Diabetic Ketoacidosis after Initiation of an SGLT2 Inhibitor. N. Engl. J. Med. 2017, 376, 2300–2302. [Google Scholar] [CrossRef]



| Variable | Level | All ICSRs (n = 2406) | ICSRs in T1DM (n = 200) | ICSRs in T2DM (n = 1191) |
|---|---|---|---|---|
| Age | <18 years (%) | 8 (0.33) | 8 (4.00) | 0 (0) |
| 18–64 years (%) | 1412 (58.69) | 118 (59.00) | 780 (65.49) | |
| >65 years (%) | 443 (18.41) | 17 (8.50) | 233 (19.56) | |
| Missing (%) | 543 (22.57) | 57 (28.50) | 178 (14.95) | |
| Gender | F (%) | 1326 (55.11) | 135 (67.50) | 623 (52.31) |
| M (%) | 1001 (41.60) | 52 (26.00) | 543 (45.59) | |
| Missing (%) | 79 (3.28) | 13 (6.50) | 25 (2.10) | |
| Seriousness of ICSR | Serious (%) | 2377 (98.79) | 197 (98.50) | 1174 (98.57) |
| Not serious (%) | 29 (1.21) | 3 (1.50) | 17 (1.43) | |
| Primary Source | Healthcare Professional (%) | 2269 (94.31) | 187 (93.50) | 1138 (95.55) |
| Non-Healthcare Professional (%) | 137 (5.69) | 13 (6.50) | 53 (4.45) | |
| Primary Source Country for Regulatory Purposes | European Economic Area (%) | 1010 (41.98) | 71 (35.50) | 547 (45.93) |
| Non-European Economic Area (%) | 1396 (58.02) | 129 (64.50) | 644 (54.07) | |
| Number of Suspected drug(s) | 1 (%) | 2069 (85,99) | 163 (81.50) | 1015 (85.22) |
| 2 (%) | 228 (9.48) | 29 (14.50) | 113 (9.49) | |
| 3 (%) | 72 (2.99) | 6 (3.00) | 39 (3.27) | |
| 4 (%) | 12 (0.50) | 1 (0.50) | 9 (0.76) | |
| ≥5 (%) | 25 (1.04) | 1 (0.50) | 15 (1.26) | |
| Number of Concomitant drug(s) | 0 (%) | 921 (38.28) | 74 (37.00) | 330 (27.71) |
| 1 (%) | 301 (12.51) | 47 (23.50) | 138 (11.59) | |
| 2 (%) | 320 (13.30) | 28 (14.00) | 181 (15.20) | |
| 3 (%) | 211 (8.77) | 20 (10.00) | 116 (9.74) | |
| 4 (%) | 143 (5.94) | 11 (5.50) | 87 (7.30) | |
| ≥5 (%) | 510 (21.20) | 20 (10.00) | 339 (28.46) |
| Variable | Level | All ketoacidosis PT (n = 2549) | Ketoacidosis PT in T1DM (n = 213) | Ketoacidosis PT in T2DM (n = 1275) |
|---|---|---|---|---|
| Seriousness | Caused/Prolonged Hospitalization (%) | 1283 (50.33) | 100 (46.95) | 664 (52.08) |
| Other Medically Important Condition (%) | 665 (26.09) | 53 (24.88) | 256 (20.08) | |
| Life Threatening (%) | 510 (20.01) | 50 (23.47) | 304 (23.84) | |
| Results in Death (%) | 45 (1.77) | 6 (2.82) | 23 (1.80) | |
| Not Serious (%) | 35 (1.37) | 4 (1.88) | 22 (1.73) | |
| Disabling (%) | 11 (0.43) | 0 (0.00) | 6 (0.47) | |
| Outcome | Recovered/Resolved (%) | 1140 (44.72) | 115 (53.99) | 652 (51.14) |
| Unknown (%) | 897 (35.19) | 64 (30.05) | 342 (26.82) | |
| Recovering/Resolving (%) | 374 (14.67) | 18 (8.45) | 204 (16.00) | |
| Not Recovered/Not Resolved (%) | 93 (3.65) | 13 (6.10) | 51 (4.00) | |
| Fatal (%) | 30 (1.18) | 2 (0.94) | 17 (1.33) | |
| Recovered/Resolved With Sequelae (%) | 15 (0.59) | 1 (0.47) | 9 (0.71) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Mauro, G.; Mascolo, A.; Gaio, M.; Rafaniello, C.; De Angelis, A.; Berrino, L.; Paolisso, G.; Rossi, F.; Capuano, A. The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study. Pharmaceuticals 2022, 15, 286. https://doi.org/10.3390/ph15030286
di Mauro G, Mascolo A, Gaio M, Rafaniello C, De Angelis A, Berrino L, Paolisso G, Rossi F, Capuano A. The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study. Pharmaceuticals. 2022; 15(3):286. https://doi.org/10.3390/ph15030286
Chicago/Turabian Styledi Mauro, Gabriella, Annamaria Mascolo, Mario Gaio, Concetta Rafaniello, Antonella De Angelis, Liberato Berrino, Giuseppe Paolisso, Francesco Rossi, and Annalisa Capuano. 2022. "The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study" Pharmaceuticals 15, no. 3: 286. https://doi.org/10.3390/ph15030286
APA Styledi Mauro, G., Mascolo, A., Gaio, M., Rafaniello, C., De Angelis, A., Berrino, L., Paolisso, G., Rossi, F., & Capuano, A. (2022). The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study. Pharmaceuticals, 15(3), 286. https://doi.org/10.3390/ph15030286