Rosmarinic Acid Attenuates the Lipopolysaccharide-Provoked Inflammatory Response of Vascular Smooth Muscle Cell via Inhibition of MAPK/NF-κB Cascade
Abstract
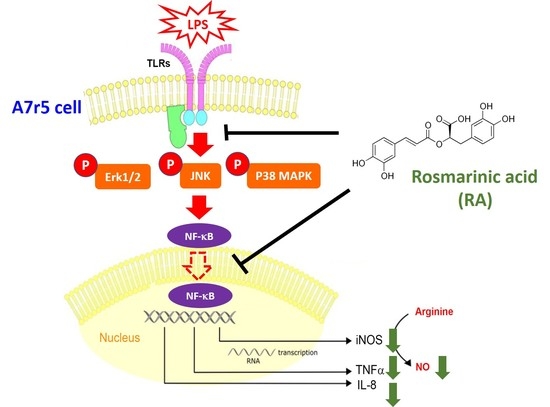
:1. Introduction
2. Results
2.1. Low-Dose RA Does Not Significantly Influence the Cell Morphology and Cell Viability of A7r5 Cells
2.2. RA Downregulated the mRNA Expression of TNFα, IL-8, and iNOS and Reduced the Production of TNFa, IL-8, and NO by A7r5 Cells
2.3. RA Inhibited the Activation of Erk, JNK, and p38 MAPK and NF-kB Signaling in A7r5 Cells in the Presence of LPS
2.4. Involvement of MAPKs and NF-kB Signaling in the RA-Inhibited Proinflammatory Response of A7r5 Cells in the Presence of LPS
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatments
4.3. Cell Viability Assay
4.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.5. Quantitation of IL-8 and TNFα Using ELISA
4.6. Nitric Oxide Production Assay
4.7. Subcellular Fractionation
4.8. Protein Extraction and Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bugger, H.; Zirlik, A. Anti-inflammatory Strategies in Atherosclerosis. Hamostaseologie 2021, 41, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Hong, H.; Zeng, D.Y.; Xie, L.N.; Cheng, Q.; Pang, X.F.; Guan, Q.G. Atorvastatin suppresses vascular hypersensitivity and remodeling induced by transient adventitial administration of lipopolysaccharide in rats. Ann. Transl. Med. 2019, 7, 386. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, S.; Hooshmand-Moghadam, B.; Rosenkranz, S.; Shourideh, Z.; Amirshaghaghi, F.; Shabkhiz, F. Improvement of inflammatory status following saffron (Crocus sativus L.) and resistance training in elderly hypertensive men: A randomized controlled trial. Exp. Gerontol. 2022, 162, 111756. [Google Scholar] [CrossRef] [PubMed]
- Malka, K.; Liaw, L. NOTCH3 as a modulator of vascular disease: A target in elastin deficiency and arterial pathologies. J. Clin. Investig. 2022, 132, e157007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, Y.; Cui, L.Y.; Tang, W.; Liu, B.; Zheng, J.J.; Si, W.Z.; Wang, X.; Xu, M.J. Suppression of Gut Bacterial Translocation Ameliorates Vascular Calcification through Inhibiting Toll-Like Receptor 9-Mediated BMP-2 Expression. Oxid. Med. Cell. Longev. 2019, 2019, 3415682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Sun, C.; Lv, X.; Sun, M.; Si, C.; Zhen, Y.; Guo, J.; Sun, W.; Ye, Z.; Wen, J.; et al. Identification of a Novel Gene Correlated With Vascular Smooth Muscle Cells Proliferation and Migration in Chronic Thromboembolic Pulmonary Hypertension. Front. Physiol. 2021, 12, 744219. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Mao, W.; Pan, L.; Sun, Y.; Fan, F.; Zhao, Y.; Cui, Y.; Wei, X.; Kohama, K.; Li, F.; et al. Inhibitory effect of recombinant human CXCL8(3-72)K11R/G31P on atherosclerotic plaques in a mouse model of atherosclerosis. Immunopharmacol. Immunotoxicol. 2019, 41, 446–454. [Google Scholar] [CrossRef]
- Ruan, H.; Huang, Q.; Wan, B.; Yang, M. Curcumin alleviates lipopolysaccharides-induced inflammation and apoptosis in vascular smooth muscle cells via inhibition of the NF-kappaB and JNK signaling pathways. Inflammopharmacology 2022, 30, 517–525. [Google Scholar] [CrossRef]
- Abekura, F.; Park, J.; Lim, H.; Kim, H.D.; Choi, H.; Lee, M.J.; Kim, C.H. Mycobacterium tuberculosis glycolipoprotein LprG inhibits inflammation through NF-kappaB signaling of ERK1/2 and JNK in LPS-induced murine macrophage cells. J. Cell. Biochem. 2022. [Google Scholar] [CrossRef]
- Kou, R.W.; Xia, B.; Wang, Z.J.; Li, J.N.; Yang, J.R.; Gao, Y.Q.; Yin, X.; Gao, J.M. Triterpenoids and meroterpenoids from the edible Ganoderma resinaceum and their potential anti-inflammatory, antioxidant and anti-apoptosis activities. Bioorg. Chem. 2022, 121, 105689. [Google Scholar] [CrossRef]
- Li, Q.; Tao, X.; Zhang, Y. Rosmarinic acid alleviates diabetic osteoporosis by suppressing the activation of NLRP3 inflammasome in rats. Physiol. Int. 2022, 109, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Hwang, B.R.; Lee, M.H.; Lee, S.; Cho, E.J. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-beta25-35 induced impairment of cognition and memory function. Nutr Res Pr. 2016, 10, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, Y.; Hitomi, Y.; Yoshida, M.; Yoshioka, E. Pharmacokinetic study of caffeic and rosmarinic acids in rats after oral administration. J. Agric. Food Chem. 2005, 53, 4740–4746. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: An evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine Int. J. Phytother. Phytopharm. 2018, 40, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yuan, J.; Zhou, N.; Shen, K.; Wang, Y.; Wang, K.; Zhu, H. Omarigliptin Prevents TNF-alpha-Induced Cellular Senescence in Rat Aorta Vascular Smooth Muscle Cells. Chem. Res. Toxicol. 2021, 34, 2024–2031. [Google Scholar] [CrossRef]
- Wang, W.J.; Cheng, M.H.; Lin, J.H.; Weng, C.S. Effect of a rosmarinic acid supplemented hemodialysis fluid on inflammation of human vascular endothelial cells. Braz. J. Med. Biol. Res. 2017, 50, e6145. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Wang, Y.; Wu, F.; Wang, C.; Wang, C.; Liu, J.; Li, P. Protective Effect of Ethyl Rosmarinate against Ulcerative Colitis in Mice Based on Untargeted Metabolomics. Int. J. Mol. Sci. 2022, 23, 1256. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Eisvand, F.; Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. In Vivo and In Vitro Protective Effects of Rosmarinic Acid against Doxorubicin-Induced Cardiotoxicity. Nutr. Cancer 2022, 74, 747–760. [Google Scholar] [CrossRef]
- Hattori, Y.; Hattori, K.; Suzuki, T.; Matsuda, N. Recent advances in the pathophysiology and molecular basis of sepsis-associated organ dysfunction: Novel therapeutic implications and challenges. Pharmaceuticals 2017, 177, 56–66. [Google Scholar] [CrossRef]
- He, Y.; Zuodong, L.; Hu, X.; Liu, X.; Gui, L.; Cai, Z.; Dai, C. Protective Effect of Panax Notoginseng Saponins on Apolipoprotein-E-deficient Atherosclerosis-prone mice. Curr. Pharm. Des. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hedayati-Moghadam, M.; Hosseinian, S.; Paseban, M.; Shabgah, A.G.; Gholizadeh, J.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Role of Chemokines in Cardiovascular Diseases and the Therapeutic Effect of Curcumin on CXCL8 and CCL2 as Pathological Chemokines in Atherosclerosis. Adv. Exp. Med. Biol. 2021, 1328, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, K.; Rica, I.; Konecna, B.; Kim, H.I.; Park, J.; Kaczmarek, E.; Hauser, C.J. Role of Mitochondria-Derived Danger Signals Released After Injury in Systemic Inflammation and Sepsis. Antioxid. Redox. Signal 2021, 35, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Su, J. Inducible Nitric Oxide Synthase (iNOS) Mediates Vascular Endothelial Cell Apoptosis in Grass Carp Reovirus (GCRV)-Induced Hemorrhage. Int. J. Mol. Sci. 2019, 20, 6335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambden, S. Bench to bedside review: Therapeutic modulation of nitric oxide in sepsis-an update. Intensive Care Med. Exp. 2019, 7, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, A.; Clark, R.F.; Hoskin, D.W.; Power Coombs, M.R. Regulation of macrophage-associated inflammatory responses by species-specific lactoferricin peptides. Front Biosci 2022, 27, 43. [Google Scholar] [CrossRef]
- Guo, H.; Li, M.; Liu, H. Selenium-Rich Yeast Peptide Fraction Ameliorates Imiquimod-Induced Psoriasis-like Dermatitis in Mice by Inhibiting Inflammation via MAPK and NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 2112. [Google Scholar] [CrossRef]
- Ouyang, Q.; Li, Y.; Mei, S.; Zhang, Q.; Li, X.; Luo, H.; Zhu, Y.; Wu, K. Protective effects of GLHP from Gracilaria lemaneiformis against UVB-induced photodamage in human immortalized keratinocytes cells and BALB/c mice. Exp. Gerontol. 2021, 155, 111550. [Google Scholar] [CrossRef]
- Haftcheshmeh, S.M.; Abedi, M.; Mashayekhi, K.; Mousavi, M.J.; Navashenaq, J.G.; Mohammadi, A.; Momtazi-Borojeni, A.A. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-kappaB, JAK/STAT, and MAPK signaling pathways. Phytother. Res. 2022. [Google Scholar] [CrossRef]
- Pipatrattanaseree, W.; Itharat, A.; Mukkasombut, N.; Saesiw, U. Potential in vitro anti-allergic, anti-inflammatory and cytotoxic activities of ethanolic extract of Baliospermum montanum root, its major components and a validated HPLC method. BMC Complement Altern. Med. 2019, 19, 45. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-P.; Lin, Y.-C.; Peng, Y.-H.; Chen, H.-M.; Lin, J.-T.; Kao, S.-H. Rosmarinic Acid Attenuates the Lipopolysaccharide-Provoked Inflammatory Response of Vascular Smooth Muscle Cell via Inhibition of MAPK/NF-κB Cascade. Pharmaceuticals 2022, 15, 437. https://doi.org/10.3390/ph15040437
Chen C-P, Lin Y-C, Peng Y-H, Chen H-M, Lin J-T, Kao S-H. Rosmarinic Acid Attenuates the Lipopolysaccharide-Provoked Inflammatory Response of Vascular Smooth Muscle Cell via Inhibition of MAPK/NF-κB Cascade. Pharmaceuticals. 2022; 15(4):437. https://doi.org/10.3390/ph15040437
Chicago/Turabian StyleChen, Ching-Pei, You-Cian Lin, Yu-Hui Peng, Han-Min Chen, Jiun-Tsai Lin, and Shao-Hsuan Kao. 2022. "Rosmarinic Acid Attenuates the Lipopolysaccharide-Provoked Inflammatory Response of Vascular Smooth Muscle Cell via Inhibition of MAPK/NF-κB Cascade" Pharmaceuticals 15, no. 4: 437. https://doi.org/10.3390/ph15040437
APA StyleChen, C. -P., Lin, Y. -C., Peng, Y. -H., Chen, H. -M., Lin, J. -T., & Kao, S. -H. (2022). Rosmarinic Acid Attenuates the Lipopolysaccharide-Provoked Inflammatory Response of Vascular Smooth Muscle Cell via Inhibition of MAPK/NF-κB Cascade. Pharmaceuticals, 15(4), 437. https://doi.org/10.3390/ph15040437