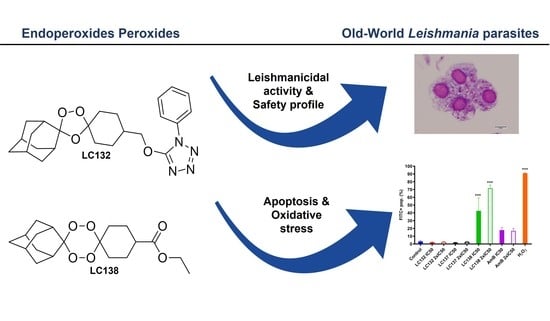
1,2,4-Trioxolane and 1,2,4,5-Tetraoxane Endoperoxides against Old-World Leishmania Parasites: In Vitro Activity and Mode of Action
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity and In Vitro Activity of Peroxidic Compounds against Leishmania sp.
2.2. Flow Cytometry Analysis
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Biological Studies
4.2.1. Parasites and Cell Lines
4.2.2. Cytotoxicity Assay
4.2.3. Studies of In Vitro Activity against Leishmania spp.
Promastigotes Susceptibility Assay
Intracellular Amastigotes Assay
4.2.4. Flow Cytometry Analysis
Detection of Phosphatidylserine
Analysis of the Mitochondrial Membrane Potential
Detection of Reactive Oxygen Species
4.2.5. Promastigotes DNA Fragmentation
4.2.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO (World Health Organization)-Leishmaniasis. Available online: https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis (accessed on 15 November 2021).
- Hendrickx, S.; Caljon, G.; Maes, L. Need for Sustainable Approaches in Antileishmanial Drug Discovery. Parasitol. Res. 2019, 118, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Copeland, N.K.; Aronson, N.E. Leishmaniasis: Treatment Updates and Clinical Practice Guidelines Review. Curr. Opin. Infect. Dis. 2015, 28, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Chetia, D. Endoperoxide Antimalarials: Development, Structural Diversity and Pharmacodynamic Aspects with Reference to 1,2,4-Trioxane-Based Structural Scaffold. Drug Des. Dev. Ther. 2016, 10, 3575–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, C.S.N.; Lam, N.S.K.; Yu, D.; Su, X.Z.; Lu, F. Artemisinin and Its Derivatives in Treating Protozoan Infections beyond Malaria. Pharmacol. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef] [Green Version]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in Vitro and in Vivo Activities of Bridged 1,2,4-Trioxolanes, Bridged 1,2,4,5-Tetraoxanes, Tricyclic Monoperoxides, Silyl Peroxides, and Hydroxylamine Derivatives against Schistosoma mansoni. Bioorganic Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef] [Green Version]
- Cortes, S.; Albuquerque, A.; Cabral, L.I.; Lopes, L.; Campino, L.; Cristiano, M.L. In Vitro Susceptibility of Leishmania infantum to Artemisinin Derivatives and Selected Trioxolanes. Antimicrob. Agents Chemother. 2015, 59, 5032–5035. [Google Scholar] [CrossRef] [Green Version]
- Sen, R.; Bandyopadhyay, S.; Dutta, A.; Mandal, G.; Ganguly, S.; Saha, P.; Chatterjee, M. Artemisinin Triggers Induction of Cell-Cycle Arrest and Apoptosis in Leishmania donovani Promastigotes. J. Med. Microbiol. 2007, 56, 1213–1218. [Google Scholar] [CrossRef] [Green Version]
- Cabral, L.I.L.; Pomel, S.; Cojean, S.; Amado, P.S.M.; Loiseau, P.M.; Cristiano, M.L.S. Synthesis and Antileishmanial Activity of 1,2,4,5-Tetraoxanes against Leishmania donovani. Molecules 2020, 25, 465. [Google Scholar] [CrossRef] [Green Version]
- Amado, P.S.M.; Frija, L.M.T.; Coelho, J.A.S.; O’Neill, P.M.; Cristiano, M.L.S. Synthesis of Non-Symmetrical Dispiro-1,2,4,5-Tetraoxanes and Dispiro-1,2,4-Trioxanes Catalyzed by Silica Sulfuric Acid. J. Org. Chem. 2021, 86, 10608–10620. [Google Scholar] [CrossRef]
- de Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Chakrabarti, S.; Staniek, K.; Gille, L.; Chatterjee, M. The Leishmanicidal Activity of Artemisinin Is Mediated by Cleavage of the Endoperoxide Bridge and Mitochondrial Dysfunction. Parasitology 2019, 146, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lobo, L.; Cabral, L.I.L.; Sena, M.I.; Guerreiro, B.; Rodrigues, A.S.; de Andrade-Neto, V.F.; Cristiano, M.L.S.; Nogueira, F. New Endoperoxides Highly Active in Vivo and in Vitro against Artemisinin-Resistant Plasmodium falciparum. Malar. J. 2018, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaire, S.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; van Bambeke, F. Study of Macrophage Functions in Murine J774 Cells and Human Activated THP-1 Cells Exposed to Oritavancin, a Lipoglycopeptide with High Cellular Accumulation. Antimicrob. Agents Chemother. 2014, 58, 2059–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista-Fernandes, T.; Marques, C.; Roos Rodrigues, O.; Santos-Gomes, G.M. Intra-Specific Variability of Virulence in Leishmania infantum Zymodeme MON-1 Strains. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 41–53. [Google Scholar] [CrossRef]
- Cortes, S.; Bruno de Sousa, C.; Morais, T.; Lago, J.; Campino, L. Potential of the Natural Products against Leishmaniasis in Old World—A Review of in-Vitro Studies. Pathog. Glob. Health 2020, 114, 170–182. [Google Scholar] [CrossRef]
- Nwaka, S.; Hudson, A. Innovative Lead Discovery Strategies for Tropical Diseases. Nat. Rev. Drug Discov. 2006, 5, 941–955. [Google Scholar] [CrossRef]
- Scariot, D.B.; Britta, E.A.; Moreira, A.L.; Falzirolli, H.; Silva, C.C.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Nakamura, C.V. Induction of Early Autophagic Process on Leishmania amazonensis by Synergistic Effect of Miltefosine and Innovative Semi-Synthetic Thiosemicarbazone. Front. Microbiol. 2017, 8, 255. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; Lopez-Velez, R. Treatment of Visceral Leishmaniasis. In The Leishmaniases: Old Neglected Tropical; Bruschi, F., Gradoni, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 169–190. ISBN 978-3-319-72386-0. [Google Scholar]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.C.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing Failure of Miltefosine in the Treatment of Kala-Azar in Nepal and the Potential Role of Parasite Drug Resistance, Reinfection, or Noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef] [Green Version]
- Corpas-López, V.; Merino-Espinosa, G.; Díaz-Sáez, V.; Morillas-Márquez, F.; Navarro-Moll, M.C.; Martín-Sánchez, J. The Sesquiterpene (−)-α-Bisabolol Is Active against the Causative Agents of Old World Cutaneous Leishmaniasis through the Induction of Mitochondrial-Dependent Apoptosis. Apoptosis 2016, 21, 1071–1081. [Google Scholar] [CrossRef]
- Saha, C. Apoptosis in Leishmania Species & Its Relevance to Disease Pathogenesis. Indian J. Med. Res. 2006, 123, 233–244. [Google Scholar]
- Menna-Barreto, R.F.S. Cell Death Pathways in Pathogenic Trypanosomatids: Lessons of (over)Kill. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, A.; Kemmer, G.; Müller, F.D.; Zampieri, R.A.; Gonzaga dos Santos, M.; Schiller, J.; Pomorski, T.G. Leishmania Promastigotes Lack Phosphatidylserine but Bind Annexin V upon Permeabilization or Miltefosine Treatment. PLoS ONE 2012, 7, e42070. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. Mitochondrial Dysfunction Indirectly Elevates ROS Production by the Endoplasmic Reticulum. Cell Metab. 2013, 18, 145–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, R.; Saha, P.; Sarkar, A.; Ganguly, S.; Chatterjee, M. Iron Enhances Generation of Free Radicals by Artemisinin Causing a Caspase-Independent, Apoptotic Death in Leishmania donovani Promastigotes. Free Radic. Res. 2010, 4, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Basmaciyan, L.; Azas, N.; Casanova, M. Different Apoptosis Pathways in Leishmania Parasites. Cell Death Discov. 2018, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Geroldinger, G.; Tonner, M.; Quirgst, J.; Walter, M.; de Sarkar, S.; Machín, L.; Monzote, L.; Stolze, K.; Catharina Duvigneau, J.; Staniek, K.; et al. Activation of Artemisinin and Heme Degradation in Leishmania tarentolae Promastigotes: A Possible Link. Biochem. Pharmacol. 2020, 173, 113737. [Google Scholar] [CrossRef]
- Vannier-Santos, M.; de Castro, S. Electron Microscopy in Antiparasitic Chemotherapy: A (Close) View to a Kill. Curr. Drug Targets 2009, 10, 246–260. [Google Scholar] [CrossRef]
- Ismael, A.; Henriques, M.S.C.; Marques, C.; Rodrigues, M.; Barreira, L.; Paixão, J.A.; Fausto, R.; Cristiano, M.L.S. Exploring Saccharinate-Tetrazoles as Selective Cu(II) Ligands: Structure, Magnetic Properties and Cytotoxicity of Copper(II) Complexes Based on 5-(3-Aminosaccharyl)-Tetrazoles. RSC Adv. 2016, 6, 71628–71637. [Google Scholar] [CrossRef]
- Kwiatkowski, M.R.; Alexanian, E.J. Nickel-Catalyzed Mizoroki–Heck-Type Reactions of Unactivated Alkyl Bromides. Angew. Chem. 2018, 130, 17099–17102. [Google Scholar] [CrossRef]
- Dutta, A.; Bandyopadhyay, S.; Mandal, C.; Chatterjee, M. Development of a Modified MTT Assay for Screening Antimonial Resistant Field Isolates of Indian Visceral Leishmaniasis. Parasitol. Int. 2005, 54, 119–122. [Google Scholar] [CrossRef]
- Maia, C.; Rolão, N.; Nunes, M.; Gonçalves, L.; Campino, L. Infectivity of Five Different Types of Macrophages by Leishmania infantum. Acta Trop. 2007, 103, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.S.; Campos, B.L.S.; Jesus, J.A.; Laurenti, M.D.; Ribeiro, S.P.; Kallás, E.G.; Rafael-Fernandes, M.; Santos-Gomes, G.; Silva, M.S.; Sessa, D.P.; et al. The Effect of Ursolic Acid on Leishmania (Leishmania) amazonensis Is Related to Programed Cell Death and Presents Therapeutic Potential in Experimental Cutaneous Leishmaniasis. PLoS ONE 2015, 10, e0144946. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cytotoxicity | Promastigote’s Susceptibility | |||||||
|---|---|---|---|---|---|---|---|---|
| CC50 ± SD (µM) | IC50 ± SD (µM) | |||||||
| Compounds | J774A.1 Cell Line | THP-1 Cell Line | L. infantum | SI (J774A.1) | SI (THP-1) | L. donovani | SI (J774A.1) | SI (THP-1) |
| LC50 | 519.3 ± 139.5 | 712.8 ± 18.4 | 378.9 ± 2.8 | 1.4 | 1.9 | 353.6 ± 8.4 | 1.5 | 2.0 |
| LC93 | 12.0 ± 0.5 | 184.9 ± 16.1 | 435.0 ± 22.7 | 0.0 | 0.4 | 152.9 ± 22.4 | 0.1 | 1.2 |
| LC129 | 323.4 ± 24.5 | 355.9 ± 31.4 | 265.9 ± 13.3 | 1.2 | 1.3 | 242,4 ± 10.2 | 1.3 | 1.5 |
| LC131 | 34.1 ± 14.5 | 78.2 ± 14.1 | 321.1 ± 11.8 | 0.1 | 0.2 | 123.5 ± 19.0 | 0.3 | 0.6 |
| LC132 | 45.8 ± 19.9 | 99.8 ± 16.4 | 13.0 ± 1.7 | 3.5 | 7.7 | 14.3 ± 5.5 | 3.2 | 7.0 |
| LC136 | 113.0 ± 13.8 | 250.5 ± 4.1 | 434.2 ± 6.4 | 0.3 | 0.6 | 231.8 ± 28.4 | 0.5 | 1.1 |
| LC137 | 443.2 ± 44.0 | 3569 ± 22 | 137.1 ± 0.2 | 3.2 | 26.0 | 143.3 ± 16.5 | 3.1 | 24.9 |
| LC138 | 527.9 ± 54.0 | 2839 ± 15 | 125.4 ± 1.6 | 4.2 | 22.6 | 239.6 ± 34.8 | 2.2 | 11.9 |
| LC139 | 912.9 ± 70.7 | 115.2 ± 11.4 | 472.3 ± 39.2 | 1.9 | 0.2 | 506.8 ± 53.3 | 1.8 | 0.2 |
| LC140 | 677.6 ± 29.9 | 1399 ± 76 | 258.2 ± 6.4 | 2.6 | 5.4 | 460.1 ± 38.3 | 1.5 | 3.0 |
| LC146 | 1465 ± 13 | 3223 ± 72 | 491.7 ± 22.7 | 3.0 | 6.6 | 793.0 ± 37.2 | 1.8 | 4.1 |
| LC163 | 474.3 ± 83.5 | 753.4 ± 90.3 | 190.3 ± 21.6 | 2.5 | 4.0 | 531.6 ± 105.2 | 0.9 | 1.4 |
| AmB | 71.9 ± 5.4 | 23.2 ± 3.0 | 0.2 ± 0.0 | 467.7 | 151.0 | 0.3 ± 0.0 | 282.5 | 91.2 |
| Miltefosine | 94.4 ± 17.3 | 100.4 ± 12.1 | 148.7 ± 3.5 | 0.6 | 0.7 | 24.2 ± 10.0 | 3.9 | 4.1 |
| Amastigote’s Susceptibility IC50 ± SD (µM) | ||||||
|---|---|---|---|---|---|---|
| Compounds | L. infantum | SI (J774A.1) | SI (THP1) | L. donovani | SI (J774A.1) | SI (THP-1) |
| LC132 | 13.2 ± 5.2 | 3.5 | 7.6 | 9.4 ± 0.1 | 4.9 | 66.3 |
| LC137 | 168.5 ± 8.9 | 2.6 | 21.2 | 88.3 ± 14.2 | 5.0 | 7.5 |
| LC138 | 23.9 ± 2.7 | 22.1 | 118.6 | 425.9 ± 25.5 | 1.2 | 10.6 |
| AmB | 0.3 ± 0.1 | 898.4 | 298.2 | 0.1 ± 0.0 | 898.4 | 40.4 |
| Miltefosine | 16.8 ± 11.7 | 5.6 | 6.0 | 13.3 ± 3.0 | 7.1 | 6.7 |
| Class | Compounds | Structures | ClogP a | Range of Concentrations (µM) | ||
|---|---|---|---|---|---|---|
| Cytotoxicity assay | Promastigote assay | Intracellular amastigote assay | ||||
| 1,2,4-trioxolanes | LC50 |  | 3.06 | 2156–33.7 | 538.9–134.7 | nd |
| LC93 |  | 3.30 | 2038–5.3 | 679.4–169.8 | nd | |
| LC129 |  | 3.86 | 1306-13.6 | 326.4–81.6 | nd | |
| LC131 |  | 3.81 | 1599–4.2 | 266.5–8.3 | nd | |
| LC132 |  | 4.05 | 1368–3.6 | 228.0–7.2 | 91.2–2.9 | |
| LC136 |  | 3.03 | 1728–46.0 | 576.0–15.3 | Nd | |
| 1,2,4,5-tetraoxanes | LC137 |  | 4.19 | 3569–66.9 | 356.9–89.2 | 285.5–8.9 |
| LC138 |  | 3.91 | 2839–53.2 | 567.9–142.0 | 425.9–13.3 | |
| LC139 |  | 2.12 | 2134–66.7 | 711.3–177.8 | nd | |
| LC140 |  | 3.04 | 3400–63.7 | 679.9–170.0 | nd | |
| LC146 |  | 3.10 | 3224–60.5 | 967.2–241.8 | nd | |
| LC163 |  | 2.94 | 2753–51.6 | 826.0–206.5 | nd | |
| AmB | 118.2–1.7 | 0.9–0.03 | 0.4–0.01 | |||
| Miltefosine | 1472–4.6 | 122.7–3.8 | 49.0–1.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, A.; Armada, A.; Cabral, L.I.L.; Amado, P.S.M.; Campino, L.; Cristiano, M.L.S.; Cortes, S. 1,2,4-Trioxolane and 1,2,4,5-Tetraoxane Endoperoxides against Old-World Leishmania Parasites: In Vitro Activity and Mode of Action. Pharmaceuticals 2022, 15, 446. https://doi.org/10.3390/ph15040446
Mendes A, Armada A, Cabral LIL, Amado PSM, Campino L, Cristiano MLS, Cortes S. 1,2,4-Trioxolane and 1,2,4,5-Tetraoxane Endoperoxides against Old-World Leishmania Parasites: In Vitro Activity and Mode of Action. Pharmaceuticals. 2022; 15(4):446. https://doi.org/10.3390/ph15040446
Chicago/Turabian StyleMendes, Andreia, Ana Armada, Lília I. L. Cabral, Patrícia S. M. Amado, Lenea Campino, Maria L. S. Cristiano, and Sofia Cortes. 2022. "1,2,4-Trioxolane and 1,2,4,5-Tetraoxane Endoperoxides against Old-World Leishmania Parasites: In Vitro Activity and Mode of Action" Pharmaceuticals 15, no. 4: 446. https://doi.org/10.3390/ph15040446
APA StyleMendes, A., Armada, A., Cabral, L. I. L., Amado, P. S. M., Campino, L., Cristiano, M. L. S., & Cortes, S. (2022). 1,2,4-Trioxolane and 1,2,4,5-Tetraoxane Endoperoxides against Old-World Leishmania Parasites: In Vitro Activity and Mode of Action. Pharmaceuticals, 15(4), 446. https://doi.org/10.3390/ph15040446