Synergistic Effect of Combination of a Temoporfin-Based Photodynamic Therapy with Potassium Iodide or Antibacterial Agents on Oral Disease Pathogens In Vitro
Abstract
:1. Introduction
2. Results
2.1. The Antimicrobial Activity of Temoporfin, KI, and the Combination of Temoporfin and KI
2.1.1. Determination of Temoporfin Treatment Conditions
2.1.2. The Kinetic Growth Curves of Temoporfin and KI Treatment in A. actinomycetemcomitans and S. mutans
2.1.3. MIC/MBC of the Temoporfin and KI Co-Treatment for Oral Bacteria under Normoxic and Hypoxic Conditions
2.2. Synergistic Effect of Temoporfin Combined with Potassium Iodide (KI)
2.3. Combination of Temoporfin with Either Ampicillin or CHX Inhibited MRSA Growth
2.4. KI Enhanced Temoporfin Biofilm Removal Effect under Hypoxic Conditions
2.4.1. The Biofilm Removal Effect of Temoporfin in a Normoxic Environment
2.4.2. The Biofilm Removal Effect of Temoporfin and KI Co-Treatment in a Hypoxic Environment
2.4.3. The Effect of Temoporfin on the Expression of Genes Regulating Biofilm Formation in MRSA
2.4.4. Endogenous Hydrogen Peroxide Production in the Species That Were Sensitive to Temoporfin and KI Co-Treatment
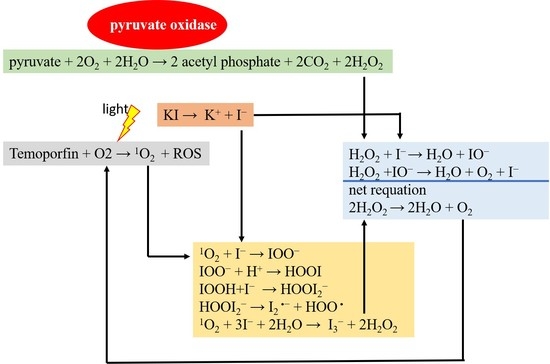
3. Discussion
4. Materials and Methods
4.1. Microorganisms Culture
4.2. Determination of Temoporfin Conditions, MIC, and MBC
4.3. Growth Curve Assay
4.4. Synergistic Effect Test
4.5. Reverse Transcription (RT)-qPCR
4.6. Biofilm Removal Assay
4.7. Hydrogen Peroxide Production Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aas Jørn, A.; Paster Bruce, J.; Stokes Lauren, N.; Olsen, I.; Dewhirst Floyd, E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar]
- Bowden, G.H.W. The microbial ecology of dental caries. Microb. Ecol. Health Dis. 2000, 12, 138–148. [Google Scholar]
- Howlin, R.P.; Fabbri, S.; Offin, D.G.; Symonds, N.; Kiang, K.S.; Knee, R.J.; Yoganantham, D.C.; Webb, J.S.; Birkin, P.R.; Leighton, T.G.; et al. Removal of dental biofilms with an ultrasonically activated water stream. J. Dent. Res. 2015, 94, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; De Jager, M.; Zaura, E.; Krom, B.P. Historical and contemporary hypotheses on the development of oral diseases: Are we there yet? Front. Cell. Infect. Microbiol. 2014, 4, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Carmona, N.; Ouk, T.S.; Leroy-Lhez, S. Latest trends on photodynamic disinfection of Gram-negative bacteria: Photosensitizer’s structure and delivery systems. Photochem. Photobiol. Sci. 2022, 21, 113–145. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, PMC.S14459. [Google Scholar] [CrossRef] [Green Version]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin Michael, R. Bacterial photodynamic inactivation mediated by methylene blue and red light Is enhanced by synergistic effect of potassium iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Mogi, M.; Okabe, I.; Okada, K.; Goto, H.; Sasaki, Y.; Fujimura, T.; Fukuda, M.; Mitani, A. Adjunctive application of antimicrobial photodynamic therapy in nonsurgical periodontal treatment: A review of literature. Int. J. Mol. Sci. 2015, 16, 24111–24126. [Google Scholar] [CrossRef] [Green Version]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef] [Green Version]
- Maisch, T. A new strategy to destroy antibiotic resistant microorganisms: Antimicrobial photodynamic treatment. Mini Rev. Med. Chem. 2009, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.T.; Dias, H.B.; Corbi, S.C.T.; Marcantonio, R.A.C.; Bernardi, A.C.A.; Bagnato, V.S.; Hamblin, M.R.; Rastelli, A.N.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016, 26, 123001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, A.; Nees, L.; Nuyts, S.; Clement, P.; Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic therapy as an alternative therapeutic tool in functionally inoperable oral and oropharyngeal carcinoma: A single tertiary center retrospective cohort analysis. Front. Oncol. 2021, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yılmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Yakavets, I.; Millard, M.; Zorin, V.; Lassalle, H.P.; Bezdetnaya, L. Current state of the nanoscale delivery systems for temoporfin-based photodynamic therapy: Advanced delivery strategies. J. Control. Release 2019, 304, 268–287. [Google Scholar] [CrossRef]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5,10,15,20-tetra(m-hydroxyphenyl)chlorin)—A second-generation photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef]
- Lange, C.; Bednarski, P.J. In vitro assessment of synergistic effects in combinations of a temoporfin-based photodynamic therapy with glutathione peroxidase 1 inhibitors. Photodiagn. Photodyn. Ther. 2021, 36, 102478. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Foster, T.H. Photophysical Parameters, Photosensitizer Retention and Tissue Optical Properties Completely Account for the Higher Photodynamic Efficacy of meso-Tetra-Hydroxyphenyl-Chlorin vs Photofrin. Photochem. Photobiol. 2005, 81, 849–859. [Google Scholar] [CrossRef]
- Sigusch, B.W.; Dietsch, S.; Berg, A.; Voelpel, A.; Guellmar, A.; Rabe, U.; Schnabelrauch, M.; Steen, D.; Gitter, B.; Albrecht, V.; et al. Antimicrobial photodynamic active biomaterials for periodontal regeneration. Dent. Mater. 2018, 34, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Ballullaya, S.V.; Thumu, J.; Maroli, S.; Shankarappa, P. Effect of ultrasonic activation of photosensitizer dye temoporfin (Foscan) on antimicrobial photodynamic therapy: An ex vivo study. J. Conserv. Dent. 2017, 20, 419–423. [Google Scholar]
- Leung, A.M.; Bauer, A.J.; Benvenga, S.; Brenner, A.V.; Hennessey, J.V.; Hurley, J.R.; Milan, S.A.; Schneider, A.B.; Sundaram, K.; Toft, D.J. American thyroid association scientific statement on the use of potassium iodide ingestion in a nuclear emergency. Thyroid 2017, 27, 865–877. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Y.; Wintner, A.; Seed, P.C.; Brauns, T.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial photodynamic therapy mediated by methylene blue and potassium iodide to treat urinary tract infection in a female rat model. Sci. Rep. 2018, 8, 7257. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Choi, H.; Kushida, Y.; Bhayana, B.; Wang, Y.; Hamblin Michael, R. Broad-spectrum antimicrobial effects of photocatalysis using titanium dioxide nanoparticles are strongly potentiated by addition of potassium iodide. Antimicrob. Agents Chemother. 2016, 60, 5445–5453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic therapy of oral candida infection in a mouse model. J. Photochem. Photobiol. B 2016, 159, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Lyu, P.; Huang, Y.Y.; Du, N.; Qi, W.; Hamblin, M.R.; Wang, Y. Potassium iodide enhances the photobactericidal effect of methylene blue on Enterococcus faecalis as planktonic cells and as biofilm infection in teeth. J. Photochem. Photobiol. B 2020, 203, 111730. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, X.; Szewczyk, G.; El-Hussein, A.; Huang, Y.Y.; Sarna, T.; Hamblin Michael, R. Potassium iodide potentiates antimicrobial photodynamic inactivation mediated by rose bengal in in vitro and in vivo studies. Antimicrob. Agents Chemother. 2017, 61, e00467-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium iodide potentiates broad-spectrum antimicrobial photodynamic inactivation using photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Laskar, N.; Kadouri, D.E. Evaluating the Effect of Oxygen Concentrations on Antibiotic Sensitivity, Growth, and Biofilm Formation of Human Pathogens. Microbiol. Insights 2016, 9, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins Antunes de Melo, W.C.; Celiesiute-Germaniene, R.; Simonis, P.; Stirke, A. Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields. Virulence 2021, 12, 2247–2272. [Google Scholar] [CrossRef] [PubMed]
- Caufield, P.W.; Schon, C.N.; Saraithong, P.; Li, Y.; Argimon, S. Oral Lactobacilli and Dental Caries: A Model for Niche Adaptation in Humans. J. Dent. Res. 2015, 94 (Suppl. 9), 110S–118S. [Google Scholar] [CrossRef]
- Huang, L.; El-Hussein, A.; Xuan, W.; Hamblin, M.R. Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J. Photochem. Photobiol. B 2018, 178, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Farinelli, G.; Minella, M.; Pazzi, M.; Giannakis, S.; Pulgarin, C.; Vione, D.; Tiraferri, A. Natural iron ligands promote a metal-based oxidation mechanism for the Fenton reaction in water environments. J. Hazard. Mater. 2020, 393, 122413. [Google Scholar] [CrossRef]
- Pesakhov, S.; Benisty, R.; Sikron, N.; Cohen, Z.; Gomelsky, P.; Khozin-Goldberg, I.; Dagan, R.; Porat, N. Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim. Biophys. Acta Biomembr. 2007, 1768, 590–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribby, S.; Taylor, M.; Reid, G. Vaginal microbiota and the use of probiotics. Interdiscip. Perspect. Infect. Dis 2008, 2008, 256490. [Google Scholar] [CrossRef]
- Chiu, K.C.; Shih, Y.H.; Wang, T.H.; Lan, W.C.; Li, P.J.; Jhuang, H.S.; Hsia, S.M.; Shen, Y.W.; Yuan-Chien Chen, M.; Shieh, T.M. In vitro antimicrobial and antipro-inflammation potential of honokiol and magnolol against oral pathogens and macrophages. J. Formos. Med. Assoc. 2021, 120, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Liu, T.C.; Mong, M.C. Antibacterial effects and action modes of asiatic acid. Biomedicine 2015, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Yu, C.C. Photodynamic therapy with 5-aminolevulinic acid (ALA) impairs tumor initiating and chemo-resistance property in head and neck cancer-derived cancer stem cells. PLoS ONE 2014, 9, e87129. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Chen, P.Y.; Ho, D.C.; Tsai, L.L.; Hsieh, P.L.; Lu, M.Y.; Yu, C.C.; Yu, C.H. miR-145 mediates the anti-cancer stemness effect of photodynamic therapy with 5-aminolevulinic acid (ALA) in oral cancer cells. J. Formos Med. Assoc. 2018, 117, 738–742. [Google Scholar] [CrossRef]
- Holowachuk, S.A.; Bal’a, M.F.; Buddington, R.K. A kinetic microplate method for quantifying the antibacterial properties of biological fluids. J. Microbiol. Methods 2003, 55, 441–446. [Google Scholar] [CrossRef]
- Wang, T.H.; Hsia, S.M.; Wu, C.H.; Ko, S.Y.; Chen, M.Y.; Shih, Y.H.; Shieh, T.M.; Chuang, L.C.; Wu, C.Y. Evaluation of the antibacterial potential of liquid and vapor phase phenolic essential oil compounds against oral microorganisms. PLoS ONE 2016, 11, e0163147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Dai, Y.; Ouyang, P.; Rehman, T.; Hussain, S.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; et al. Thymol inhibits biofilm formation, eliminates pre-existing biofilms, and enhances clearance of methicillin-resistant staphylococcus aureus (MRSA) in a mouse peritoneal implant infection model. Microorganisms 2020, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Normoxia (MIC/MBC) (μg/mL) | Hypoxia (MIC/MBC) (μg/mL) | |||
|---|---|---|---|---|
| Oral Bacteria | Temoporfin | Temoporfin + 1 mg/mL KI | Temoporfin | Temoporfin + 1 mg/mL KI |
| A. actinomycetemcomitans (G−) | 4/4 | 4/4 | 4/8 | 2/4 |
| E. faecalis (G+) | 1/1 | 1/1 | 1/1 | 1/2 |
| L. acidophilus (G+) | >8/>8 | 2/4 | >8/>8 | 2/4 |
| L. paracasei (G+) | >8/>8 | 2/3 | >8/>8 | 2/3 |
| S. aureus (G+) | 1/2 | 1/1 | 2/2 | 1/1 |
| MRSA (G+) | 8/8 | 4/8 | 8/8 | 4/8 |
| S. mutans (G+) | 2/2 | 0.5/1 | 2/2 | 2/2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, Y.-H.; Yu, C.-C.; Chang, K.-C.; Tseng, Y.-H.; Li, P.-J.; Hsia, S.-M.; Chiu, K.-C.; Shieh, T.-M. Synergistic Effect of Combination of a Temoporfin-Based Photodynamic Therapy with Potassium Iodide or Antibacterial Agents on Oral Disease Pathogens In Vitro. Pharmaceuticals 2022, 15, 488. https://doi.org/10.3390/ph15040488
Shih Y-H, Yu C-C, Chang K-C, Tseng Y-H, Li P-J, Hsia S-M, Chiu K-C, Shieh T-M. Synergistic Effect of Combination of a Temoporfin-Based Photodynamic Therapy with Potassium Iodide or Antibacterial Agents on Oral Disease Pathogens In Vitro. Pharmaceuticals. 2022; 15(4):488. https://doi.org/10.3390/ph15040488
Chicago/Turabian StyleShih, Yin-Hwa, Cheng-Chia Yu, Kai-Chi Chang, Yu-Hsin Tseng, Po-Jung Li, Shih-Min Hsia, Kuo-Chou Chiu, and Tzong-Ming Shieh. 2022. "Synergistic Effect of Combination of a Temoporfin-Based Photodynamic Therapy with Potassium Iodide or Antibacterial Agents on Oral Disease Pathogens In Vitro" Pharmaceuticals 15, no. 4: 488. https://doi.org/10.3390/ph15040488
APA StyleShih, Y. -H., Yu, C. -C., Chang, K. -C., Tseng, Y. -H., Li, P. -J., Hsia, S. -M., Chiu, K. -C., & Shieh, T. -M. (2022). Synergistic Effect of Combination of a Temoporfin-Based Photodynamic Therapy with Potassium Iodide or Antibacterial Agents on Oral Disease Pathogens In Vitro. Pharmaceuticals, 15(4), 488. https://doi.org/10.3390/ph15040488