Rapid Absorption of Naloxone from Eye Drops
Abstract
:1. Introduction
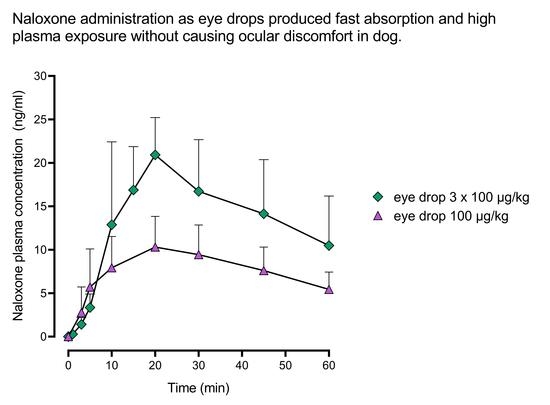
2. Results
2.1. Pharmacokinetics
2.2. Clinical Signs and Local Tolerability
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. The First Pharmacokinetic Study—Dose Finding
4.4. The Second Pharmacokinetic Study—Formulation Comparison
4.5. Drug Administration and Blood Sampling
4.6. Naloxone Plasma Concentration Analysis
4.7. Clinical Signs and Ocular Tolerability
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dezfulian, C.; Orkin, A.M.; Maron, B.A.; Elmer, J.; Girotra, S.; Gladwin, M.T.; Merchant, R.M.; Panchal, A.R.; Perman, S.M.; Starks, M.A.; et al. Opioid-associated out-of-hospital cardiac arrest: Distinctive clinical features and implications for health care and public responses: A scientific statement from the American Heart Association. Circulation 2021, 143, e836–e870. [Google Scholar] [CrossRef] [PubMed]
- CDC. Drug Overdose Deaths; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/drugoverdose/data/statedeaths.html (accessed on 28 March 2022).
- CDC. Suspected Nonfatal Drug Overdoses during COVID-19; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/drugoverdose/data/nonfatal/states/covid-19.html (accessed on 28 March 2022).
- Moss, R.B.; Carlo, D.J. Higher doses of naloxone are needed in the synthetic opiod era. Subst. Abuse Treat. Prev. Policy 2019, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. FDA Approves Higher Dosage of Naloxone Nasal Spray to Treat Opioid Overdose. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose (accessed on 28 March 2022).
- European Monitoring Centre for Drugs and Drug Addiction. Preventing Opioid Overdose Deaths with Take-Home Naloxone. 2016. Available online: https://www.emcdda.europa.eu/publications/insights/take-home-naloxone_en (accessed on 28 March 2022).
- Moustaqim-Barrette, A.; Dhillon, D.; Ng, J.; Sundvick, K.; Ali, F.; Elton-Marshall, T.; Leece, P.; Rittenbach, K.; Ferguson, M.; Buxton, J.A. Take-home naloxone programs for suspected opioid overdose in community settings: A scoping umbrella review. BMC Public Health 2021, 21, 597. [Google Scholar] [CrossRef] [PubMed]
- Strang, J.; McDonald, R.; Alqurshi, A.; Royall, P.; Taylor, D.; Forbes, B. Naloxone without the needle–systematic review of candidate routes for non-injectable naloxone for opioid overdose reversal. Drug Alcohol Depend. 2016, 163, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urtti, A.; Salminen, L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv. Ophthalmol. 1993, 37, 435–456. [Google Scholar] [CrossRef]
- Docci, L.; Umehara, K.; Krähenbühl, S.; Fowler, S.; Parrott, N. Construction and verification of physiologically based pharmacokinetic models for four drugs majorly cleared by glucuronidation: Lorazepam, oxazepam, naloxone, and zidovudine. AAPS J. 2020, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- FDA. December 17–18, 2018: Joint Meeting of the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee Meeting Announcement. 2018. Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar/december-17-18-2018-joint-meeting-anesthetic-and-analgesic-drug-products-advisory-committee-and-drug (accessed on 28 March 2022).
- FDA. Statement from FDA Commissioner Scott Gottlieb, M.D., on Unprecedented New Efforts to Support Development of Over-the-Counter Naloxone to Help Reduce Opioid Overdose Deaths. 2019. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-unprecedented-new-efforts-support-development-over (accessed on 28 March 2022).
- Hamor, R.E.; Roberts, S.M.; Severin, G.A.; Chavkin, M.J. Evaluation of results for Schirmer tear tests conducted with and without application of a topical anesthetic in clinically normal dogs of 5 breeds. Am. J. Vet. Res. 2000, 61, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, H.J.; Heinrich, C.L. Ophthalmic examination and diagnostics. In Veterinary Ophthalmology, 6th ed.; Gelatt, K.N., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; Volume 1, pp. 602–603. [Google Scholar]
- Sebbag, L.; Kirner, N.S.; Allbaugh, R.A.; Reis, A.; Mochel, J.P. Kinetics of fluorescein in tear film after eye drop instillation in beagle dogs: Does size really matter? Front. Vet. Sci. 2019, 6, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebbag, L.; Mochel, J.P. An eye on the dog as the scientist’s best friend for translational research in ophthalmology: Focus on the ocular surface. Med. Res. Rev. 2020, 40, 2566–2604. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G.; Narayan, R.J. Naloxone and nalmefene absorption delivered by hollow microneedles compared to intramuscular injection. Drug Deliv. Transl. Res. 2022, 12, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Wahler, B.M.; Lerche, P.; Pereira, C.H.R.; Bednarski, R.M.; KuKanich, B.; Lakritz, J.; Aarnes, T.K. Pharmacokinetics and pharmacodynamics of intranasal and intravenous naloxone hydrochloride administration in healthy dogs. Am. J. Vet. Res. 2019, 80, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Krieter, P.A.; Chiang, C.N.; Gyaw, S.; McCann, D.J. Comparison of the pharmacokinetic properties of naloxone following the use of FDA-approved intranasal and intramuscular devices versus a common improvised nasal naloxone device. J. Clin. Pharmacol. 2019, 59, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Krieter, P.; Chiang, N.; Gyaw, S.; Skolnick, P.; Crystal, R.; Keegan, F.; Aker, J.; Beck, M.; Harris, J. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J. Clin. Pharmacol. 2016, 56, 1243–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Finnish Government. Act on the Protection of Animals Used for Scientific or Educational Purposes (497/2013). Available online: https://www2.helsinki.fi/sites/default/files/atoms/files/act_in_english_2014.pdf?msclkid=83cee446c45611ecb59144d1cd2a2e06 (accessed on 28 March 2022).
- Finnish Government. Decree on the Protection of Animals Used for Scientific or Educational Purposes (564/2013). Available online: https://www.finlex.fi/en/laki/kaannokset/2013/en20130564.pdf?msclkid=89c10a85c45711ec90fc87472618a941 (accessed on 28 March 2022).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79.





| Dosing Route | Dose | Cmax ng/mL | tmax h | AUClast h × ng/mL | tlast h | BA % | |
|---|---|---|---|---|---|---|---|
| µg/kg | µL/kg | ||||||
| Intravenous | 100 | 6.7 | 33.5 ± 5.3 | 7.0 ± 2.0 | |||
| Ocular | 20 | 6.7 | 2.2 ± 0.5 | 0.38 ± 0.22 | 3.1 ± 1.2 | 5.0 ± 1.1 | 37 ± 10 |
| Ocular | 100 | 6.7 | 12.2 ± 2.9 | 0.36 ± 0.24 | 15.4 ± 5.0 | 7.7 ± 0.8 | 41 ± 10 |
| Ocular | 3 × 100 | 3 × 6.7 | 23.5 ± 4.6 | 0.32 ± 0.11 | 28.4 ± 11.0 | 8.0 ± 0.0 | 29 ± 10 |
| Dosing Route | Vehicle | Dose | Cmax ng/mL | tmax h | AUClast h × ng/mL | tlast h | BA % | |
|---|---|---|---|---|---|---|---|---|
| µg/kg | µL/kg | |||||||
| Intravenous | Saline | 100 | 6.7 | 27.9 ± 7.5 | 2.0 ± 0.0 | |||
| Ocular | Citrate buffer | 100 | 6.7 | 12.7 ± 1.3 | 0.46 ± 0.10 | 16.5 ± 5.3 | 3.0 ± 1.1 | 56 ± 14 |
| Ocular | PG + EDTA | 100 | 6.7 | 10.3 * ± 1.8 | 0.28 ± 0.11 | 11.3 * ± 3.0 | 2.7 ± 1.0 | 45 ± 10 |
| Ocular | PG + EDTA in citrate buffer | 100 | 6.7 | 11.2 ± 2.4 | 0.23 ± 0.16 | 10.9 * ± 2.4 | 2.0 ± 0.8 | 48 ± 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuunainen, J.; Saloranta, L.; Levijoki, J.; Lindstedt, J.; Lehtisalo, J.; Pappinen, S.; Ramela, M.; Virtanen, S.; Joensuu, H. Rapid Absorption of Naloxone from Eye Drops. Pharmaceuticals 2022, 15, 532. https://doi.org/10.3390/ph15050532
Tuunainen J, Saloranta L, Levijoki J, Lindstedt J, Lehtisalo J, Pappinen S, Ramela M, Virtanen S, Joensuu H. Rapid Absorption of Naloxone from Eye Drops. Pharmaceuticals. 2022; 15(5):532. https://doi.org/10.3390/ph15050532
Chicago/Turabian StyleTuunainen, Johanna, Lasse Saloranta, Jouko Levijoki, Jenni Lindstedt, Jenni Lehtisalo, Sari Pappinen, Meri Ramela, Sami Virtanen, and Heikki Joensuu. 2022. "Rapid Absorption of Naloxone from Eye Drops" Pharmaceuticals 15, no. 5: 532. https://doi.org/10.3390/ph15050532
APA StyleTuunainen, J., Saloranta, L., Levijoki, J., Lindstedt, J., Lehtisalo, J., Pappinen, S., Ramela, M., Virtanen, S., & Joensuu, H. (2022). Rapid Absorption of Naloxone from Eye Drops. Pharmaceuticals, 15(5), 532. https://doi.org/10.3390/ph15050532