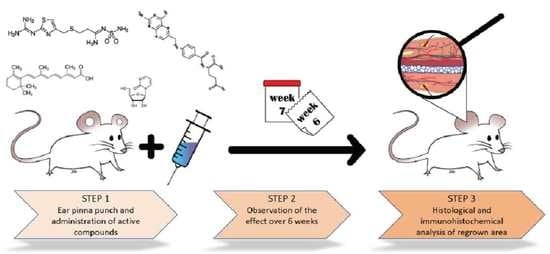
Regenerative Drug Discovery Using Ear Pinna Punch Wound Model in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Delayed Zebularine Delivery
2.2. Modifying Zebularine Effects with Small-Molecule Bio-Active Compounds
2.2.1. Immunomodulators
2.2.2. Methyl Donors
2.3. Testing Retinoids and Vitamin D3 in the Ear Punch Wound Model
2.4. Diet and Ear Pinna Hole Closure
2.5. Testing Non-Nucleoside Epigenetic Inhibitors in the Ear Punch Wound Model
2.6. Impact of Mouse Age on Ear Pinna Healing
2.7. Correlations of Healing between Left and Right Ears
2.8. Nerve Fibres and Vessels in Regenerating Ear Pinnae
3. Materials and Methods
3.1. Animals
3.2. Ear Pinna Punch Wound Experiment
3.3. Fortified Diet Experiment
3.4. Immunohistochemical Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlile, S. The auditory periphery of the ferret. II: The spectral transformations of the external ear and their implications for sound localization. J. Acoust. Soc. Am. 1990, 88, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. The Human Body: Linking Structure and Function; Academic Press: London, UK, 2018. [Google Scholar]
- Qin, W.; Wang, R.K. Assessment of edema volume in skin upon injury in a mouse ear model with optical coherence tomography. Lasers Med. Sci. 2016, 31, 1351–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonanno, M.; Randers-Pehrson, G.; Smilenov, L.; Kleiman, N.; Young, E.; Ponnayia, B.; Brenner, D. A mouse ear model for bystander studies induced by microbeam irradiation. Radiat. Res. 2015, 184, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potez, M.; Bouchet, A.; Wagner, J.; Donzelli, M.; Bräuer-Krisch, E.; Hopewell, J.W.; Laissue, J.; Djonov, V. Effects of synchrotron x-ray micro-beam irradiation on normal mouse ear pinnae. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, T.; Li, W.; Yang, L.; Li, P.; Cao, H.; Motegi, S.-I.; Udey, M.C.; Bernhard, E.; Nakamura, T.; Mukouyama, Y.-S. Whole-mount adult ear skin imaging reveals defective neuro-vascular branching morphogenesis in obese and type 2 diabetic mouse models. Sci. Rep. 2018, 8, 430. [Google Scholar] [CrossRef] [Green Version]
- Barker, J.H.; Hammersen, F.; Bondar, I.; Uhl, E.; Galla, T.J.; Menger, M.D.; Messmer, K. The hairless mouse ear for in vivo studies of skin microcirculation. Plast. Reconstr. Surg. 1989, 83, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J.; Grimes, L.N. Tissue interactions in the regeneration of rabbit ear holes. Am. Zool. 1972, 12, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Kloeters, O.; Tandara, A.; Mustoe, T.A. Hypertrophic scar model in the rabbit ear: A reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007, 15, S40–S45. [Google Scholar] [CrossRef]
- Clark, L.D.; Clark, R.K.; Heber-Katz, E. A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 1998, 88, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Blankenhorn, E.P.; Troutman, S.; Clark, L.D.; Zhang, X.-M.; Chen, P.; Heber-Katz, E. Sexually dimorphic genes regulate healing and regeneration in MRL mice. Mamm. Genome 2003, 14, 250–260. [Google Scholar] [CrossRef]
- Buckley, G.; Metcalfe, A.D.; Ferguson, M.W. Peripheral nerve regeneration in the MRL/MpJ ear wound model. J. Anat. 2011, 218, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.C.; Mustoe, T.A. Animal models of wound healing: Uility in transgenic mice. J. Biomater. Sci. Polym. Ed. 2008, 19, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikova, E.; McIntosh, D.; Dewilde, A.; Zhang, J.; Reing, J.E.; Zhang, L.; Cordero, K.; Bedelbaeva, K.; Gourevitch, D.; Heber-Katz, E. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010, 29, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Muneoka, K. The blastema and epimorphic regeneration in mammals. Dev. Biol. 2018, 433, 190–199. [Google Scholar] [CrossRef]
- Kumar, A.; Brockes, J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef]
- Gawronska-Kozak, B. Regeneration in the ears of immunodeficient mice: Identification and lineage analysis of mesenchymal stem cells. Tissue Eng. 2004, 10, 1251–1265. [Google Scholar] [CrossRef]
- Bedelbaeva, K.; Snyder, A.; Gourevitch, D.; Clark, L.; Zhang, X.-M.; Leferovich, J.; Cheverud, J.M.; Lieberman, P.; Heber-Katz, E. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 5845–5850. [Google Scholar] [CrossRef] [Green Version]
- Oike, Y.; Yasunaga, K.; Ito, Y.; Matsumoto, S.-I.; Maekawa, H.; Morisada, T.; Arai, F.; Nakagata, N.; Takeya, M.; Masuho, Y. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 9494–9499. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Strehin, I.; Bedelbaeva, K.; Gourevitch, D.; Clark, L.; Leferovich, J.; Messersmith, P.B.; Heber-Katz, E. Drug-induced regeneration in adult mice. Sci. Transl. Med. 2015, 7, 290ra92. [Google Scholar] [CrossRef] [Green Version]
- Bastakoty, D.; Saraswati, S.; Cates, J.; Lee, E.; Nanney, L.B.; Young, P.P. Inhibition of Wnt/β-catenin pathway promotes regenerative repair of cutaneous and cartilage injury. FASEB J. 2015, 29, 4881–4892. [Google Scholar] [CrossRef] [Green Version]
- Leferovich, J.M.; Bedelbaeva, K.; Samulewicz, S.; Zhang, X.-M.; Zwas, D.; Lankford, E.B.; Heber-Katz, E. Heart regeneration in adult MRL mice. Proc. Natl. Acad. Sci. USA 2001, 98, 9830–9835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuret, S.; Thallmair, M.; Horky, L.L.; Gage, F.H. Enhanced functional recovery in MRL/MpJ mice after spinal cord dorsal hemisection. PLoS ONE 2012, 7, e30904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalley, A.L.; Dyment, N.A.; Kazemi, N.; Kenter, K.; Gooch, C.; Rowe, D.W.; Butler, D.L.; Shearn, J.T. Improved biomechanical and biological outcomes in the MRL/MpJ murine strain following a full-length patellar tendon injury. J. Orthop. Res. 2015, 33, 1693–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, M.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Shaffer, D.J.; Roopenian, D.C.; Shultz, L.D. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4097–4106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Krebs, M.P.; Kaushal, S.; Scott, E.W. Enhanced retinal pigment epithelium regeneration after injury in MRL/MpJ mice. Exp. Eye Res. 2011, 93, 862–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.; Sinha, K.; Pan, H.; Cui, Y.; Guo, P.; Lin, C.Y.; Yang, F.; Deng, Z.; Eltzschig, H.K.; Lu, A. Markers of accelerated skeletal muscle regenerative response in Murphy Roths large mice: Characteristics of muscle progenitor cells and circulating factors. Stem Cells 2019, 37, 357–367. [Google Scholar] [CrossRef]
- Chadwick, R.B.; Bu, L.; Yu, H.; Hu, Y.; Wergedal, J.E.; Mohan, S.; Baylink, D.J. Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice. Wound Repair Regen. 2007, 15, 275–284. [Google Scholar] [CrossRef]
- Seifert, A.W.; Kiama, S.G.; Seifert, M.G.; Goheen, J.R.; Palmer, T.M.; Maden, M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012, 489, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Górnikiewicz, B.; Ronowicz, A.; Podolak, J.; Madanecki, P.; Stanisławska-Sachadyn, A.; Sachadyn, P. Epigenetic basis of regeneration: Analysis of genomic DNA methylation profiles in the MRL/MpJ mouse. DNA Res. 2013, 20, 605–621. [Google Scholar] [CrossRef] [Green Version]
- Podolak-Popinigis, J.; Ronowicz, A.; Dmochowska, M.; Jakubiak, A.; Sachadyn, P. The methylome and transcriptome of fetal skin: Implications for scarless healing. Epigenomics 2016, 8, 1331–1345. [Google Scholar] [CrossRef]
- Górnikiewicz, B.; Ronowicz, A.; Madanecki, P.; Sachadyn, P. Genome-wide DNA methylation profiling of the regenerative MRL/MpJ mouse and two normal strains. Epigenomics 2017, 9, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Górnikiewicz, B.; Ronowicz, A.; Krzemiński, M.; Sachadyn, P. Changes in gene methylation patterns in neonatal murine hearts: Implications for the regenerative potential. BMC Genom. 2016, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, P.; Sosnowski, P.; Podolak-Popinigis, J.; Górnikiewicz, B.; Kamińska, J.; Deptuła, M.; Nowicka, E.; Wardowska, A.; Ruczyński, J.; Rekowski, P. Epigenetic inhibitor zebularine activates ear pinna wound closure in the mouse. EBioMedicine 2019, 46, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nittby, H.; Ericsson, P.; Förnvik, K.; Strömblad, S.; Jansson, L.; Xue, Z.; Skagerberg, G.; Widegren, B.; Sjögren, H.-O.; Salford, L.G. Zebularine induces long-term survival of pancreatic islet allotransplants in streptozotocin treated diabetic rats. PLoS ONE 2013, 8, e71981. [Google Scholar] [CrossRef] [Green Version]
- Sass, P.A.; Dąbrowski, M.; Charzyńska, A.; Sachadyn, P. Transcriptomic responses to wounding: Meta-analysis of gene expression microarray data. BMC Genom. 2017, 18, 850. [Google Scholar] [CrossRef] [Green Version]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Toll, E.C.; Seifalian, A.M.; Birchall, M.A. The role of immunophilin ligands in nerve regeneration. Regen. Med. 2011, 6, 635–652. [Google Scholar] [CrossRef]
- Ducruet, A.F.; DeRosa, P.A.; Zacharia, B.E.; Sosunov, S.A.; Connolly, E.S., Jr.; Weinstein, D.E. GM1485, a nonimmunosuppressive immunophilin ligand, promotes neurofunctional improvement and neural regeneration following stroke. J. Neurosci. Res. 2012, 90, 1413–1423. [Google Scholar] [CrossRef]
- Cheng, J.C.; Yoo, C.B.; Weisenberger, D.J.; Chuang, J.; Wozniak, C.; Liang, G.; Marquez, V.E.; Greer, S.; Orntoft, T.F.; Thykjaer, T. Preferential response of cancer cells to zebularine. Cancer Cell 2004, 6, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, B.J.; Rizk, E.; Meier, B.; Hariharan, N.; Bottiglieri, T.; Finnell, R.H.; Jarrard, D.F.; Banerjee, R.V.; Skene, J.P.; Nelson, A. Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J. Clin. Investig. 2010, 120, 1603–1616. [Google Scholar] [CrossRef]
- Chu, D.; Li, L.; Jiang, Y.; Tan, J.; Ji, J.; Zhang, Y.; Jin, N.; Liu, F. Excess folic acid supplementation before and during pregnancy and lactation activates Fos gene expression and alters behaviors in male mouse offspring. Front. Neurosci. 2019, 13, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, I.C.; Champagne, F.A.; Brown, S.E.; Dymov, S.; Sharma, S.; Meaney, M.J.; Szyf, M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: Altering epigenetic marking later in life. J. Neurosci. 2005, 25, 11045–11054. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A. Assessing the effects of high methionine intake on DNA methylation. J. Nutr. 2006, 136, 1706S–1710S. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef] [Green Version]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic acid and its derivatives in skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef]
- Wong, M.S.K.; Leisegang, M.S.; Kruse, C.; Vogel, J.; Schürmann, C.; Dehne, N.; Weigert, A.; Herrmann, E.; Brüne, B.; Shah, A.M. Vitamin D promotes vascular regeneration. Circulation 2014, 130, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Chabas, J.-F.; Stephan, D.; Marqueste, T.; Garcia, S.; Lavaut, M.-N.; Nguyen, C.; Legre, R.; Khrestchatisky, M.; Decherchi, P.; Feron, F. Cholecalciferol (vitamin D3) improves myelination and recovery after nerve injury. PLoS ONE 2013, 8, e65034. [Google Scholar] [CrossRef] [Green Version]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 463. [Google Scholar] [CrossRef]
- Pijnappel, W.; Hendriks, H.; Folkers, G.; van den Brink, C.; Dekker, E.; Edelenbosch, C.; van der Saag, P.; Durston, A. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature 1993, 366, 340–344. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Budavari, S. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck and Co. Inc.: Whitehouse Station, NJ, USA, 2001; Volume 767, p. 4342. [Google Scholar]
- Leo, M.A.; Lieber, C.S. Alcohol, vitamin A, and β-carotene: Adverse interactions, including hepatotoxicity and carcinogenicity. Am. J. Clin. Nutr. 1999, 69, 1071–1085. [Google Scholar] [CrossRef] [Green Version]
- Veraldi, S.; Rossi, L.C.; Barbareschi, M. Are topical retinoids teratogenic? G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2016, 151, 700–705. [Google Scholar]
- Podolak-Popinigis, J.; Górnikiewicz, B.; Ronowicz, A.; Sachadyn, P. Transcriptome profiling reveals distinctive traits of retinol metabolism and neonatal parallels in the MRL/MpJ mouse. BMC Genom. 2015, 16, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jara, C.P.; Mendes, N.F.; do Prado, T.P.; de Araújo, E.P. Bioactive fatty acids in the resolution of chronic inflammation in skin wounds. Adv. Wound Care 2020, 9, 472–490. [Google Scholar] [CrossRef] [PubMed]
- Russell, L. The importance of patients’ nutritional status in wound healing. Br. J. Nurs. 2001, 10, S42–S49. [Google Scholar] [CrossRef]
- Ben-Kasus, T.; Ben-Zvi, Z.; Marquez, V.E.; Kelley, J.A.; Agbaria, R. Metabolic activation of zebularine, a novel DNA methylation inhibitor, in human bladder carcinoma cells. Biochem. Pharmacol. 2005, 70, 121–133. [Google Scholar] [CrossRef]
- Herranz, M.; Martín-Caballero, J.; Fraga, M.F.; Ruiz-Cabello, J.; Flores, J.M.; Desco, M.; Marquez, V.; Esteller, M. The novel DNA methylation inhibitor zebularine is effective against the development of murine T-cell lymphoma. Blood 2006, 107, 1174–1177. [Google Scholar] [CrossRef]
- Lee, G.; Wolff, E.; Miller, J.H. Mutagenicity of the cytidine analog zebularine in Escherichia coli. DNA Repair 2004, 3, 155–161. [Google Scholar] [CrossRef]
- Orta, M.L.; Pastor, N.; Burgos-Morón, E.; Domínguez, I.; Calderón-Montaño, J.M.; Castaño, C.H.; López-Lázaro, M.; Helleday, T.; Mateos, S. Zebularine induces replication-dependent double-strand breaks which are preferentially repaired by homologous recombination. DNA Repair 2017, 57, 116–124. [Google Scholar] [CrossRef]
- Brueckner, B.; Boy, R.G.; Siedlecki, P.; Musch, T.; Kliem, H.C.; Zielenkiewicz, P.; Suhai, S.; Wiessler, M.; Lyko, F. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005, 65, 6305–6311. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.-H.; Cheng, C.-C.; Chen, Y.-C.; Chung, C.-C.; Lee, T.-I.; Chen, S.-A.; Chen, Y.-J. Hydralazine-induced promoter demethylation enhances sarcoplasmic reticulum Ca2+-ATPase and calcium homeostasis in cardiac myocytes. Lab. Investig. 2011, 91, 1291–1297. [Google Scholar] [CrossRef]
- Tampe, B.; Steinle, U.; Tampe, D.; Carstens, J.L.; Korsten, P.; Zeisberg, E.M.; Müller, G.A.; Kalluri, R.; Zeisberg, M. Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury–to–chronic kidney disease progression. Kidney Int. 2017, 91, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.M.; Mahar, M.; Ewan, E.E.; Leahy, K.M.; Zhao, G.; Cavalli, V. Epigenetic regulator UHRF1 inactivates REST and growth suppressor gene expression via DNA methylation to promote axon regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E12417–E12426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Zahoor, M.; Hwang, J.-K.; Min, D.S.; Choi, K.-Y. Valproic acid induces cutaneous wound healing in vivo and enhances keratinocyte motility. PLoS ONE 2012, 7, e48791. [Google Scholar] [CrossRef]
- Cui, S.-S.; Yang, C.P.; Bowen, R.C.; Bai, O.; Li, X.-M.; Jiang, W.; Zhang, X. Valproic acid enhances axonal regeneration and recovery of motor function after sciatic nerve axotomy in adult rats. Brain Res. 2003, 975, 229–236. [Google Scholar] [CrossRef]
- Reines, B.; Cheng, L.I.; Matzinger, P. Unexpected regeneration in middle-aged mice. Rejuvenation Res. 2009, 12, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flurkey, K.; Currer, J.M.; Harrison, D. Mouse models in aging research. In The Mouse in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2007; pp. 637–672. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-S.; Kurpad, D.S.; Mahoney, M.G.; Steinbeck, M.J.; Freeman, T.A. Inhibition of apoptosis signal-regulating kinase 1 alters the wound epidermis and enhances auricular cartilage regeneration. PLoS ONE 2017, 12, e0185803. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Driever, P.H.; Sander, J.W. Cancer risk in people with epilepsy: The role of antiepileptic drugs. Brain 2005, 128, 7–17. [Google Scholar] [CrossRef] [Green Version]














| Vitamins | Standard Maintenance Feed C1320 (Altromin) (mg/kg Feed) | Unsaturated Fatty Acids-Enriched Feed (UFA Feed) C1057 (Altromin) (mg/kg Feed) | Vitamins Added (mg/kg Feed) | Vitamin-Fortified UFA Feed (mg/kg Feed) | Fortification vs. Standard Diet % |
|---|---|---|---|---|---|
| Vitamin A | 4.5 * | 4.5 * | 15 | 19.5 | 433% |
| Vitamin B5 | 21 | 50 | 600 | 650 | 3095% |
| Vitamin C | 36 | 20 | 5000 | 5020 | 13,944% |
| Vitamin D3 | 0.015 ** | 0.0125 ** | 1.25 | 1.2625 | 8417% |
| Compound | Source | Cat. No. |
|---|---|---|
| All-trans 4-keto retinoic acid | TRC (Toronto Research Chemicals, Toronto, Canada) | K204980 |
| Desloratadine | TCI Europe (Tokyo Chemical Industry, Zwijndrecht, Belgium) | D3787 |
| Famotidine | TCI Europe (Tokyo Chemical Industry, Zwijndrecht, Belgium) | F0530 |
| Folic acid | Sigma-Aldrich (Poznań, Poland) | F7876 |
| GM1485 | Key Organics (Camelford, UK) | EG-0058 |
| Hydralazine | TCI Europe (Tokyo Chemical Industry, Zwijndrecht, Belgium) | H0409 |
| L-5-methyltetrahydrofolate | Biosynth Carbosynth (Staad, Switzerland) | FM11406 |
| Methionine | Sigma-Aldrich (Poznań, Poland) | M5308 |
| All-trans-retinoic acid | TCI Europe (Tokyo Chemical Industry, Zwijndrecht, Belgium) | R0064 |
| RG108 | Synthesis by P. Mucha, University of Gdańsk (Supplementary File S2) | |
| Tacrolimus | Selleckchem (Houston, TX, U.S.A.) | S5003 |
| Valproic acid | TCI Europe (Tokyo Chemical Industry, Zwijndrecht, Belgium) | S0894 |
| Vitamin B5 (D-pantothenic acid) | Sigma-Aldrich (Poznań, Poland) | 21210 |
| Vitamin C (L-ascorbic acid) | Sigma-Aldrich (Poznań, Poland) | A0278 |
| Vitamin D3 (cholecalciferol) | TCI Europe (Tokyo Chemical Industry, Zwijndrecht, Belgium) | C0314 |
| Zebularine | TCI Europe (Tokyo Chemical Industry, Zwijndrecht, Belgium) | Z0022 |
| Compound | Dose | Vehicles | Volume | Admin. Schedule (Injection Days) |
|---|---|---|---|---|
| Zebularine | 1000 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 * |
| Zebularine | 200 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Saline control | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 | |
| RG108 | 10 mg/kg b.w. | Saline + 1% DMSO | 0.2 mL | 0–4, 7–10 |
| Control for RG108 | Saline + 1% DMSO | 0.2 mL | 0–4, 7–10 | |
| All-trans-retinoic acid | 16 mg/kg b.w. | Rapeseed oil + 10% DMSO | 0.2 mL | 0–4, 7–11 or 0, 2, 4, 7, 9, 11, 14, 16, 18 ** |
| Control for retinoids | Rapeseed oil + 10% DMSO | 0.2 mL | 0–4, 7, 11 | |
| Hydralazine | 10 mg/kg b.w. | Saline | 0.01 mL per gram b.w. | 0–4, 7, 10 |
| Valproic acid | 25 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| 500 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 | |
| Famotidine | 0.5 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Desloratadine | 0.5 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Tacrolimus | 0.25 mg/kg b.w. | Saline | 0.4 mL | 0–4, 7, 11 |
| GM1485 | 5 mg/kg b.w. | Saline | 0.2 mL | 0–4, 7, 11 |
| Famotidine + zebularine | 0.5 mg/kg b.w. + 1000 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Desloratadine + zebularine | 0.5 mg/kg b.w. + 1000 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Zebularine + folic acid + L-5-methyltetra-hydrofolate | 1000 mg/kg b.w. + 0.16 mg/kg b.w. + 0.08 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Folic acid + L-5-methyltetra-hydrofolate | 0.16 mg/kg b.w. 0.08 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Folic acid | 0.08 mg/kg b.w. | Saline | 0.2 mL | 0–4, 7–11 |
| L-5-methyltetra-hydrofolate | 0.08 mg/kg b.w. | Saline | 0.2 mL | 0–4, 7–11 |
| Vitamin C (ascorbic acid) | 1% | Saline | 0.2 mL | 0–4, 7–11 |
| Methionine | 125 mg/kg b.w. | Saline | 0.02 mL per gram b.w. | 0–4, 7, 10 |
| Vitamin D3 | 50 IU | Rapeseed oil | 0.1 mL | 0–4, 7–11 |
| Zebularine + Retinoic acid *** | 1000 mg/kg b.w. 16 mg/kg b.w | Saline Rapeseed oil, 0.3% DMSO | 0.02 mL per gram b.w. 0.2 mL | 0–4, 7, 10 0, 2, 4, 7,9, 11 |
| Control for zebularine and retinoic acid *** | Saline Rapeseed oil, 0.3% DMSO | 0.02 mL per gram b.w. 0.2 mL | 0–4, 7, 10 0, 2, 4, 7,9, 11 |
| Antibody | Marker | Conjugated | Host | Clonality and Isotype | Supplier, Cat. Number |
|---|---|---|---|---|---|
| Tuj1 | III β-tubulin | Alexa Fluor 647 | Mouse | Monoclonal, IgG2a | Biolegend, 801201 |
| αSMA | Alfa smooth muscle actin | Cy3 | Mouse | Monoclonal, IgG2a | Merck, C6198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnowski, P.; Sass, P.; Słonimska, P.; Płatek, R.; Kamińska, J.; Baczyński Keller, J.; Mucha, P.; Peszyńska-Sularz, G.; Czupryn, A.; Pikuła, M.; et al. Regenerative Drug Discovery Using Ear Pinna Punch Wound Model in Mice. Pharmaceuticals 2022, 15, 610. https://doi.org/10.3390/ph15050610
Sosnowski P, Sass P, Słonimska P, Płatek R, Kamińska J, Baczyński Keller J, Mucha P, Peszyńska-Sularz G, Czupryn A, Pikuła M, et al. Regenerative Drug Discovery Using Ear Pinna Punch Wound Model in Mice. Pharmaceuticals. 2022; 15(5):610. https://doi.org/10.3390/ph15050610
Chicago/Turabian StyleSosnowski, Paweł, Piotr Sass, Paulina Słonimska, Rafał Płatek, Jolanta Kamińska, Jakub Baczyński Keller, Piotr Mucha, Grażyna Peszyńska-Sularz, Artur Czupryn, Michał Pikuła, and et al. 2022. "Regenerative Drug Discovery Using Ear Pinna Punch Wound Model in Mice" Pharmaceuticals 15, no. 5: 610. https://doi.org/10.3390/ph15050610
APA StyleSosnowski, P., Sass, P., Słonimska, P., Płatek, R., Kamińska, J., Baczyński Keller, J., Mucha, P., Peszyńska-Sularz, G., Czupryn, A., Pikuła, M., Piotrowski, A., Janus, Ł., Rodziewicz-Motowidło, S., Skowron, P., & Sachadyn, P. (2022). Regenerative Drug Discovery Using Ear Pinna Punch Wound Model in Mice. Pharmaceuticals, 15(5), 610. https://doi.org/10.3390/ph15050610