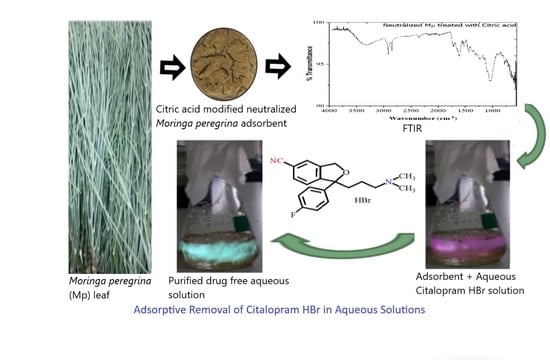
Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of pH on the Adsorption
2.2. Optimization of Variables
2.2.1. Fitting of the Model and Relevant Statistical Analysis
2.2.2. Effect of the Adsorption Parameters on the Responses
2.2.3. Numerical Optimization Using the Desirability Function
2.3. Adsorption Isotherms
2.4. Adsorption Thermodynamics
2.5. Adsorption Kinetic Model
2.6. Kinetic Adsorption Mechanism
2.6.1. Intraparticle Diffusion Model
2.6.2. Diffusion-Chemisorption Model
2.7. Comparison with other adsorbents
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Standards
3.3. Synthesis of Chemically Modified Moringa peregrina Leaf Adsorbent
3.4. Experimental Design for Optimization
3.5. Adsorption Procedure
3.6. Desirability Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, D. The pharmaceutical industry and the future of drug development. In Pharmaceuticals in the Environment; Hester, R.E., Harrison, R.M., Eds.; RSC: London, UK, 2015; pp. 1–33. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- Domingo-Echaburu, S.; Lertxundi, U.; Boxall, A.B.; Orive, G. Environmental contamination by pet pharmaceuticals: A hidden problem. Sci. Total Environ. 2021, 788, 147827. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Sun, J.; Wang, W.; Yang, L.; Xu, Y. Pharmaceuticals in two watersheds in Eastern China and their ecological risks. Environ. Pollut. 2021, 277, 116773. [Google Scholar] [CrossRef]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Mathew, R.A.; Kanmani, S. A review on emerging contaminants in indian waters and their treatment technologies. Nat. Environ. Pollut. Technol. 2020, 19, 549–562. [Google Scholar] [CrossRef]
- Gu, Q.; Dillon, C.; Burt, V. Prescription drug use continues to increase: US prescription drug data for 2007–2008. NCHS Data Brief. 2010, 42, 1–8. [Google Scholar]
- Lam, D.; Gorman, D.A.; Patten, S.; Pringsheim, T. The pharmacoepidemiology of selective serotonin reuptake inhibitors for children and adolescents in Canada from 2005 to 2009: A database analysis. Pediatr. Drugs 2013, 15, 319–327. [Google Scholar] [CrossRef]
- Styrishave, B.; Halling-Sørensen, B.; Ingerslev, F. Environmental risk assessment of three selective serotonin reuptake inhibitors in the aquatic environment: A case study including a cocktail scenario. Environ. Toxicol. Chem. 2011, 30, 254–261. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vajda, A.M. Antidepressant pharmaceuticals in two US effluent-impacted streams: Occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef]
- Cunha, D.L.; de Araujo, F.G.; Marques, M. Psychoactive drugs: Occurrence in aquatic environment, analytical methods, and ecotoxicity—A review. Environ. Sci. Pollut. Res. 2017, 24, 24076–24091. [Google Scholar] [CrossRef]
- Subedi, B.; Balakrishna, K.; Sinha, R.K.; Yamashita, N.; Balasubramanian, V.G.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products, including psychoactive and illicit drugs and artificial sweeteners, in five sewage treatment plants in India. J. Environ. Chem. Eng. 2015, 3, 2882–2891. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.G.; Catalá, M.; Maroto, R.R.; Gil, J.L.R.; de Miguel, Á.G.; Valcárcel, Y. Pollution by psychoactive pharmaceuticals in the Rivers of Madrid metropolitan area (Spain). Environ. Int. 2010, 36, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ruan, Y.; Lin, H.; Yuen, C.N.; Feng, H.; Lam, P.K. Occurrence and fate of psychiatric pharmaceuticals in wastewater treatment plants in Hong Kong: Enantiomeric profiling and preliminary risk assessment. ACS EST Water 2020, 1, 542–552. [Google Scholar] [CrossRef]
- Buřič, M.; Grabicová, K.; Kubec, J.; Kouba, A.; Kuklina, I.; Kozák, P.; Grabic, R.; Randák, T. Environmentally relevant concentrations of tramadol and citalopram alter behaviour of an aquatic invertebrate. Aquat. Toxicol. 2018, 200, 226–232. [Google Scholar] [CrossRef]
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barceló, D.; Iqbal, H.N.; Parra-Saldívar, R. Antidepressant drugs as emerging contaminants: Occurrence in urban and non-urban waters and analytical methods for their detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Li, N. Oxidation of citalopram with sodium hypochlorite and chlorine dioxide: Influencing factors and NDMA formation kinetics. Molecules 2019, 24, 3065. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Holgado, C.; Calza, P.; Fabbri, D.; Dal Bello, F.; Medana, C.; Sakkas, V. Investigation of the aquatic photolytic and photocatalytic degradation of citalopram. Molecules 2021, 26, 5331. [Google Scholar] [CrossRef]
- Arola, K.; Hatakka, H.; Mänttäri, M.; Kallioinen, M. Novel process concept alternatives for improved removal of micropollutants in wastewater treatment. Sep. Purif. Technol. 2017, 186, 333–341. [Google Scholar] [CrossRef]
- Bergersen, O.; Hanssen, K.; Vasskog, T. Anaerobic treatment of sewage sludge containing selective serotonin reuptake inhibitors. Bioresour. Technol. 2012, 117, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, O.; Hanssen, K.Ø.; Vasskog, T. Aerobic treatment of selective serotonin reuptake inhibitors in landfill leachate. Environ. Sci. Eur. 2015, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Zhou, L.; Liu, Z.; Heng, J.Y.; Chen, W. Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: A review. Sep. Purif. Technol. 2020, 253, 117536. [Google Scholar] [CrossRef]
- Calisto, V.; Ferreira, C.I.; Santos, S.M.; Gil, M.V.; Otero, M.; Esteves, V.I. Production of adsorbents by pyrolysis of paper mill sludge and application on the removal of citalopram from water. Bioresour. Technol. 2014, 166, 335–344. [Google Scholar] [CrossRef]
- Sharifabadi, M.K.; Tehrani, M.S.; Mehdinia, A.; Azar, P.A.; Husain, S.W. Fast removal of citalopram drug from waste water using magnetic nanoparticles modified with sodium dodecyl sulfate followed by UV-spectrometry. J. Chem. Health Risks 2013, 3, 35–41. [Google Scholar]
- Masud, A.; Soria, N.G.C.; Aga, D.S.; Aich, N. Adsorption and advanced oxidation of diverse pharmaceuticals and personal care products (PPCPs) from water using highly efficient rGO–nZVI nanohybrids. Environ. Sci. Water Res. Technol. 2020, 6, 2223–2238. [Google Scholar] [CrossRef]
- König, R.; Spaggiari, M.; Santoliquido, O.; Principi, P.; Bianchi, G.; Ortona, A. Micropollutant adsorption from water with engineered porous ceramic architectures produced by additive manufacturing and coated with natural zeolite. J. Clean Prod. 2020, 258, 120500. [Google Scholar] [CrossRef]
- Zhiteneva, V.; Ziemendorf, É.; Sperlich, A.; Drewes, J.E.; Hübner, U. Differentiating between adsorption and biodegradation mechanisms while removing trace organic chemicals (TOrCs) in biological activated carbon (BAC) filters. Sci. Total Environ. 2020, 743, 140567. [Google Scholar] [CrossRef]
- Rahman, N.; Nasir, M. N-(((2-((2-Aminoethyl) amino) ethyl) amino) methyl)-4-sulfamoylbenzamide impregnated hydrous zirconium oxide as a novel adsorbent for removal of Ni (II) from aqueous solutions: Optimization of variables using central composite design. ACS Omega 2019, 4, 2823–2832. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.; Nasir, M. Effective removal of acetaminophen from aqueous solution using Ca (II)-doped chitosan/β-cyclodextrin composite. J. Mol. Liq. 2020, 301, 112454. [Google Scholar] [CrossRef]
- Rahman, N.; Varshney, P. Effective removal of doxycycline from aqueous solution using CuO nanoparticles decorated poly (2-acrylamido-2-methyl-1-propanesulfonic acid)/chitosan. Environ. Sci. Pollut. Res. 2021, 28, 43599–43617. [Google Scholar] [CrossRef] [PubMed]
- Carrington, R.; Hall, M.C. Carboxylated Cellulose Ion-Exchange Materials. U.S. Patent US4260740A, 7 April 1981. [Google Scholar]
- Altundoğan, H.S.; Topdemir, A.; Cakmak, M.; Bahar, N. Hardness removal from waters by using citric acid modified pine cone. J. Taiwan Inst. Chem. Eng. 2016, 58, 219–225. [Google Scholar] [CrossRef]
- Yan, J.; Lan, G.; Qiu, H.; Chen, C.; Liu, Y.; Du, G.; Zhang, J. Adsorption of heavy metals and methylene blue from aqueous solution with citric acid modified peach stone. Sep. Sci. Technol. 2018, 53, 1678–1688. [Google Scholar] [CrossRef] [Green Version]
- Bellostas, N.; Soslash, J.C.; Nikiema, A.; Soslash, H.; Pasternak, D.; Kumar, S. Glucosinolates in leaves of Moringa species grown and disseminated in Niger. Afr. J. Agric. Res. 2010, 5, 1338–1340. [Google Scholar]
- Al-Owaisi, M.; Al-Hadiwi, N.; Khan, S.A. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac. J. Trop. Biomed. 2014, 4, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Meneghel, A.P.; Gonçalves, A.C.; Rubio, F.; Dragunski, D.C.; Lindino, C.A.; Strey, L. Biosorption of cadmium from water using Moringa (Moringa oleifera Lam.) seeds. Water Air Soil. Pollut. 2013, 224, 1–13. [Google Scholar] [CrossRef]
- Putzig, C.L.; Leugers, M.A.; McKelvy, M.L.; Mitchell, G.E.; Nyquist, R.A.; Papenfuss, R.R.; Yurga, L. Infrared spectroscopy. Anal. Chem. 1994, 66, 26R–66R. [Google Scholar] [CrossRef]
- Han, R.; Zhang, L.; Song, C.; Zhang, M.; Zhu, H.; Zhang, L. Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr. Polym. 2010, 79, 1140–1149. [Google Scholar] [CrossRef]
- Nabais, J.V.; Ledesma, B.; Laginhas, C. Removal of amitriptyline from aqueous media using activated carbons. Adsorp. Sci. Technol. 2012, 30, 255–263. [Google Scholar] [CrossRef]
- Marshall, W.E.; Wartelle, L.H. Chromate (CrO42−) and copper (Cu2+) adsorption by dual-functional ion exchange resins made from agricultural by-products. Water Res. 2006, 40, 2541–2548. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.; Varshney, P. Assessment of ampicillin removal efficiency from aqueous solution by polydopamine/zirconium (IV) iodate: Optimization by response surface methodology. RSC Adv. 2020, 10, 20322–20337. [Google Scholar] [CrossRef] [PubMed]
- Lach, J. Adsorption of chloramphenicol on commercial and modified activated carbons. Water 2019, 11, 1141. [Google Scholar] [CrossRef] [Green Version]
- Azmi, S.N.H.; Al-Balushi, M.; Al-Siyabi, F.; Al-Hinai, N.; Khurshid, S. Adsorptive removal of Pb (II) ions from groundwater samples in Oman using carbonized Phoenix dactylifera seed (Date stone). J. King Saud. Univ. Sci. 2020, 32, 2931–2938. [Google Scholar] [CrossRef]
- Saleh, T.A. Isotherm, kinetic, and thermodynamic studies on Hg (II) adsorption from aqueous solution by silica-multiwall carbon nanotubes. Environ. Sci. Pollut. Res. 2015, 22, 16721–16731. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Jemutai-Kimosop, S. Efficient removal of sulfamethoxazole onto sugarcane bagasse-derived biochar: Two and three-parameter isotherms, kinetics and thermodynamics. S. Afr. J. Chem. 2020, 73, 111–119. [Google Scholar]
- Togue Kamga, F. Modeling adsorption mechanism of paraquat onto Ayous (Triplochiton scleroxylon) wood sawdust. Appl. Water Sci. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Weber Jr, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- To, M.-H.; Hadi, P.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Mechanistic study of atenolol, acebutolol and carbamazepine adsorption on waste biomass derived activated carbon. J. Mol. Liq. 2017, 241, 386–398. [Google Scholar] [CrossRef]
- Rahman, N.; Nasir, M. Facile synthesis of thiosalicylic acid functionalized silica gel for effective removal of Cr (III): Equilibrium modeling, kinetic and thermodynamic studies. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100353. [Google Scholar] [CrossRef]
- Rahman, N.; Sameen, S.; Kashif, M. Application of Box-Behnken design and desirability function in the optimization of spectrophotometric method for the quantification of WADA banned drug: Acetazolamide. J. Mol. Liq. 2019, 274, 270–277. [Google Scholar] [CrossRef]









| Experimental Run | A: Time (min) | B: pH | C: Adsorbent Dose (g) | Observed Response (%) | Predicted Response (%) |
|---|---|---|---|---|---|
| 1 | 4 | 11 | 0.02 | 19.00 | 19.27 |
| 2 | 4 | 7 | 0.17 | 82.59 | 82.59 |
| 3 | 7.5 | 7 | 0.32 | 72.84 | 72.84 |
| 4 | 0.5 | 11 | 0.17 | 53.09 | 53.08 |
| 5 | 0.5 | 3 | 0.17 | 24.32 | 24.29 |
| 6 | 4 | 7 | 0.17 | 82.59 | 82.59 |
| 7 | 7.5 | 11 | 0.17 | 56.72 | 56.97 |
| 8 | 4 | 11 | 0.32 | 70.13 | 70.13 |
| 9 | 4 | 3 | 0.02 | 7.87 | 7.85 |
| 10 | 4 | 3 | 0.32 | 28.23 | 28.25 |
| 11 | 0.5 | 7 | 0.32 | 59.75 | 59.76 |
| 12 | 7.5 | 7 | 0.02 | 29.89 | 29.89 |
| 13 | 4 | 7 | 0.17 | 82.59 | 82.59 |
| 14 | 4 | 7 | 0.17 | 82.59 | 82.59 |
| 15 | 0.5 | 7 | 0.02 | 31.22 | 31.23 |
| 16 | 4 | 7 | 0.17 | 82.59 | 82.59 |
| 17 | 7.5 | 3 | 0.17 | 32.47 | 32.47 |
| Model | SD | F | p-Values | R2 | Adjusted R2 | Predicted R2 | PRESS |
|---|---|---|---|---|---|---|---|
| Linear | 159.24 | 2.44 | 0.0189 | 0.2127 | 0.6767 | 0.4890 | 7.61 × 105 |
| Two-factor interaction | 167.90 | 0.14 | 0.1450 | 0.0185 | 0.6405 | −0.6754 | 2.450 × 106 |
| Quadratic | 13.73 | 6.40 × 108 | <0.0001 | 1.0000 | 0.9999 | 0.9999 | 1951.71 |
| Isotherm * | Parameters | R2 | Error Function (χ2) | |
|---|---|---|---|---|
| Langmuir | qe (mg·g−1) | 8.55 | 0.999 | 3.24 × 10−3 |
| KL (L·mg−1) | 0.08 | |||
| RL | 0.26 | |||
| Freundlich | qe (mg·g−1) | 8.41 | 0.996 | 3.77 × 10−2 |
| 1/n | 1.53 | |||
| Kf | 0.51 | |||
| Temkin | qe (mg·g−1) | 8.30 | 0.919 | 6.54 × 10−1 |
| AT (Lg−1) | 0.49 | |||
| BT (J·mol−1) | 7.56 | |||
| Dubinin–Radushkevich | qe (mg·g−1) | 8.20 | 0.926 | 1.04 |
| Kad (mol2·J−2) | −2 × 10−6 | |||
| E (kJ·mol−1) | −0.50 | |||
| Kinetic Model | Parameters | ||
|---|---|---|---|
| Pseudo first order | qe (mg·g−1) | k1 (min−1) | R2 |
| 2.59 | 0.63 | 0.9975 | |
| Pseudo second order | qe (mg·g−1) | k2 (g mg−1 min−1) | R2 |
| 8.50 | 0.69 | 0.9995 | |
| Intraparticle diffusion | Cid (mg·g−1) | Kid (mg·g−1 min−1/2) | R2 |
| 6.44 | 1.16 | 0.9810 | |
| Diffusion chemisorption | qe (mg·g−1) | Kdc (mg·g−1 min−1/2) | R2 |
| 8.50 | 98.04 | 0.9995 | |
| Adsorbent | Contact Time (min) | Removal (%) | Adsorption Capacity (mg·g−1) | Reference |
|---|---|---|---|---|
| Paper mill sludge pyrolyzed at 800 ℃ | 30 | - | 8.50 | [25] |
| SDS-coated magnetic particles | 10 | 79.80 | - | [26] |
| Reduced graphene oxide (rGO) | 10 | 26.30 | - | [27] |
| Nanoscale zero valent iron (nZVI) | 10 | 31.41 | - | [27] |
| rGO/nZVI | 10 | 47.90 | - | [27] |
| # Porous alumina coated with natural zeolite | - | (i) 84.00 (ii) 75.00 (iii) 82.00 | - | [28] |
| Citric-acid-modified cellulose adsorbent derived from Moringa peregrina leaf | 4 | 82.59 | 8.58 | This work |
| Variables | Unit | Factor | Range and Level | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Contact time | minute | A | 0.50 | 4.00 | 7.50 |
| pH | B | 3.00 | 7.00 | 11.00 | |
| Adsorbent dose | g | C | 0.02 | 0.17 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azmi, S.N.H.; Al Lawati, W.M.; Al Hoqani, U.H.A.; Al Aufi, E.; Al Hatmi, K.; Al Zadjali, J.S.; Rahman, N.; Nasir, M.; Rahman, H.; Khan, S.A. Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions. Pharmaceuticals 2022, 15, 760. https://doi.org/10.3390/ph15060760
Azmi SNH, Al Lawati WM, Al Hoqani UHA, Al Aufi E, Al Hatmi K, Al Zadjali JS, Rahman N, Nasir M, Rahman H, Khan SA. Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions. Pharmaceuticals. 2022; 15(6):760. https://doi.org/10.3390/ph15060760
Chicago/Turabian StyleAzmi, Syed Najmul Hejaz, Wafa Mustafa Al Lawati, Umaima Hamed Abdullah Al Hoqani, Ekhlas Al Aufi, Khalsa Al Hatmi, Jumana Salim Al Zadjali, Nafisur Rahman, Mohd Nasir, Habibur Rahman, and Shah A. Khan. 2022. "Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions" Pharmaceuticals 15, no. 6: 760. https://doi.org/10.3390/ph15060760
APA StyleAzmi, S. N. H., Al Lawati, W. M., Al Hoqani, U. H. A., Al Aufi, E., Al Hatmi, K., Al Zadjali, J. S., Rahman, N., Nasir, M., Rahman, H., & Khan, S. A. (2022). Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions. Pharmaceuticals, 15(6), 760. https://doi.org/10.3390/ph15060760